Photo AI
Last Updated Sep 24, 2025
Intermolecular Forces Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Intermolecular Forces quickly and effectively.
383+ students studying
Intermolecular Forces
Introduction
Chemical structures are crucial in explaining why substances behave uniquely under different conditions. They determine how substances interact, dissolve, or conduct electricity. Grasping this foundation is vital in chemistry.
Types of Chemical Structures
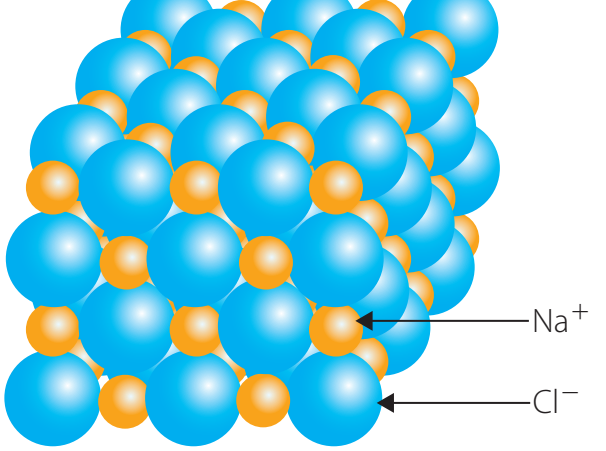
Ionic Networks
Ionic Networks: Composed of ions arranged in a lattice through electrostatic attraction. This is essential to understanding properties such as high melting points and brittleness in salts like sodium chloride.
- Characteristics:
- Typically formed by elements in Groups 1 and 17.
- Arranged in a structured lattice.
- Evident in compounds like table salt.
Covalent Networks
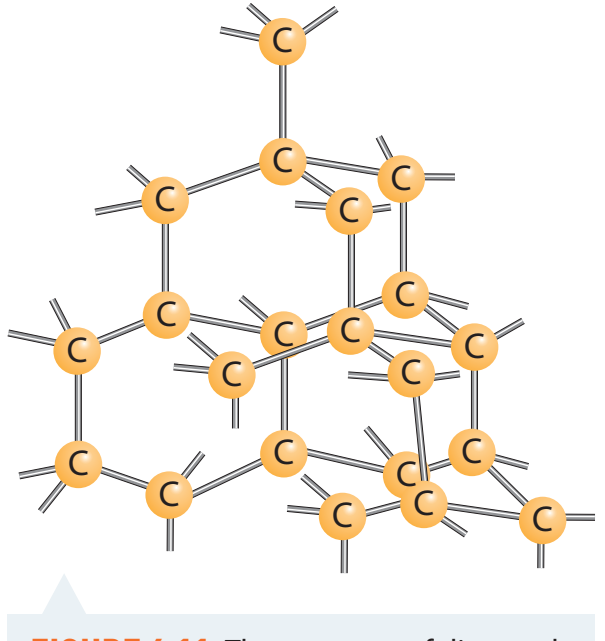
Covalent Networks: Formed by strong covalent bonds creating an extensive lattice, leading to high melting points and structural strength. Diamond is a well-known example of its durability, used in cutting tools.
- Characteristics:
- High melting points.
- Hardness.
- Examples: Diamond (used for cutting) and quartz.
Covalent Molecular Structures
Covalent Molecular Structures: Consist of discrete molecules with weak intermolecular forces, which account for the low boiling points of substances like water, explaining why they exist as liquids.
- Characteristics:
- Low boiling points.
- Example: Water is essential for life, remaining liquid due to its structure.

Metallic Structures
Metallic Structures: Metals with a 'sea of electrons', contributing to flexibility and high conductivity. This concept explains why metals are used in electrical wires and components.
- Characteristics:
- High electrical conductivity.
- Malleability.
- Common examples include copper and iron wiring in electronics.

Ionic Networks in Detail
Ionic Network: A three-dimensional lattice structure where ions are interconnected through ionic bonds.
- Ionic Bonding: Electrostatic attraction between oppositely charged ions, releasing energy and forming stable structures.
- Examples:
- NaCl: Common salt, present in food and seawater.
- MgO: Known for high melting point, used in industrial applications like refractories.
Ionic Network: A configuration where ions form a consistent lattice, impacting melting and boiling points.
Arrangement in a Lattice Structure
- Crystalline Lattice: Formed by ionic bonding; provides structural stability.
- Organised Structure:
- Property: High Melting Point
- Explanation: Strong ionic bonds reinforce the structure.
- Property: Brittleness
- Explanation: Rigid lattice is prone to shatter under stress.
- Property: High Melting Point
Covalent Networks Explained
Definitions and Characteristics
- Covalent Networks: Large, stable structures formed from continuous covalent bonds.
- Characteristics:
- High Melting Points: Requires significant energy.
- Insolubility: Typically insoluble in water due to strong, non-polar bonds.
- Electrical Conductivity: Generally poor, except graphite.
- Hardness: Especially pronounced in diamonds.
- Characteristics:
Graphite allows electron movement between layers, contributing to its distinctive electrical properties.
Intermolecular and Intramolecular Forces
Definitions
Intermolecular Forces: Forces that occur between molecules, crucial for determining physical properties such as boiling and melting points. They are key in understanding how substances interact and change.
Intramolecular Forces: Strong forces that hold atoms together within a molecule, including ionic, covalent, and metallic bonds. These determine the basic chemical properties of substances.
Types of Intermolecular Forces
- Van der Waals Forces
- Weak forces due to temporary dipoles.
- Dipole-Dipole Interactions
- Present in polar molecules.
- Hydrogen Bonding
- Strong dipole type found in water, elevating boiling points.
Metallic Structures and the Sea of Electrons
Concept Introduction: The Sea of Electrons concept describes freely moving electrons within the metal lattice.
- Electrical Conductivity: Efficient conduction due to electron mobility.
- Malleability and Ductility: Electron cloud enables atomic layers to slide over each other.
Comparative Overview
- Comparison of Structures:
| Property | Ionic | Covalent Network | Covalent Molecular | Metallic |
|---|---|---|---|---|
| Bonding | Ionic Bonds | Covalent Bonds | Intermolecular Forces | Metallic Bonds |
| Melting Points | High | High | Low | Variable |
| Conductivity | Low | Low | Low | High |
- Examples:
- NaCl (Ionic): Conducts electricity when molten.
- Diamond (Covalent): Hard due to strong covalent bonds.
- Water (Covalent Molecular): High boiling point from hydrogen bonding.
- Copper (Metallic): Conducts electricity, malleable due to electron sea.
Understanding these forces helps in designing engineering materials.
Worked Example: Comparing Boiling Points
Problem: Explain why the boiling point of water (H₂O, 100°C) is much higher than methane (CH₄, -162°C) despite both having similar molecular masses.
Solution:
- Water molecules form hydrogen bonds between the oxygen atom of one molecule and the hydrogen atom of another.
- Methane molecules only interact via weak van der Waals forces.
- Hydrogen bonds are much stronger than van der Waals forces, requiring more energy to break.
- Therefore, water needs more heat energy to boil, resulting in a higher boiling point.
![Diagram illustrating hydrogen bonding between water molecules vs. van der Waals forces between methane molecules]
Conclusion: The presence of hydrogen bonding in water significantly increases its boiling point compared to methane, which only has weak van der Waals forces.
Allotropy
Allotropy refers to elements existing in different structural forms while in the same physical state. Carbon provides excellent examples:
-
Diamond
- Each carbon atom forms four covalent bonds in a tetrahedral arrangement
- Results in an extremely hard, rigid structure
- Poor electrical conductor as all electrons are used in bonding
-
Graphite
- Carbon atoms form three covalent bonds in planar hexagonal rings
- Layers can slide over each other (responsible for lubricating properties)
- Good electrical conductor due to delocalized electrons between layers
The different properties of diamond and graphite demonstrate how the same element can exhibit vastly different characteristics based on its structure.
500K+ Students Use These Powerful Tools to Master Intermolecular Forces For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
197 flashcards
Flashcards on Intermolecular Forces
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Intermolecular Forces
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes8 questions
Exam questions on Intermolecular Forces
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Intermolecular Forces
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Intermolecular Forces
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Intermolecular Forces you should explore
Discover More Revision Notes Related to Intermolecular Forces to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Intermolecular Forces and Allotropy
Carbon's Different Forms
440+ studying
182KViews96%
114 rated
Intermolecular Forces and Allotropy
Electronegativity and Bond Polarity
292+ studying
190KViews