Photo AI
Last Updated Sep 24, 2025
Electronegativity and Bond Polarity Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Electronegativity and Bond Polarity quickly and effectively.
218+ students studying
Electronegativity and Bond Polarity
Definition of Electronegativity
Electronegativity: The tendency of an atom to attract electrons within a chemical bond. This fundamental concept aids in understanding atomic interactions.
- Linus Pauling Scale: Used for quantifying and comparing the electronegativity of elements.
- Significance: Understanding electronegativity facilitates predictions regarding bond types and the chemical reactivity of various elements.
Pauling Scale Values:
- Fluorine: 3.98
- Oxygen: 3.44
- Chlorine: 3.16
- Sodium: 0.93
- The Linus Pauling Scale is essential for comprehending element interactions in chemistry.
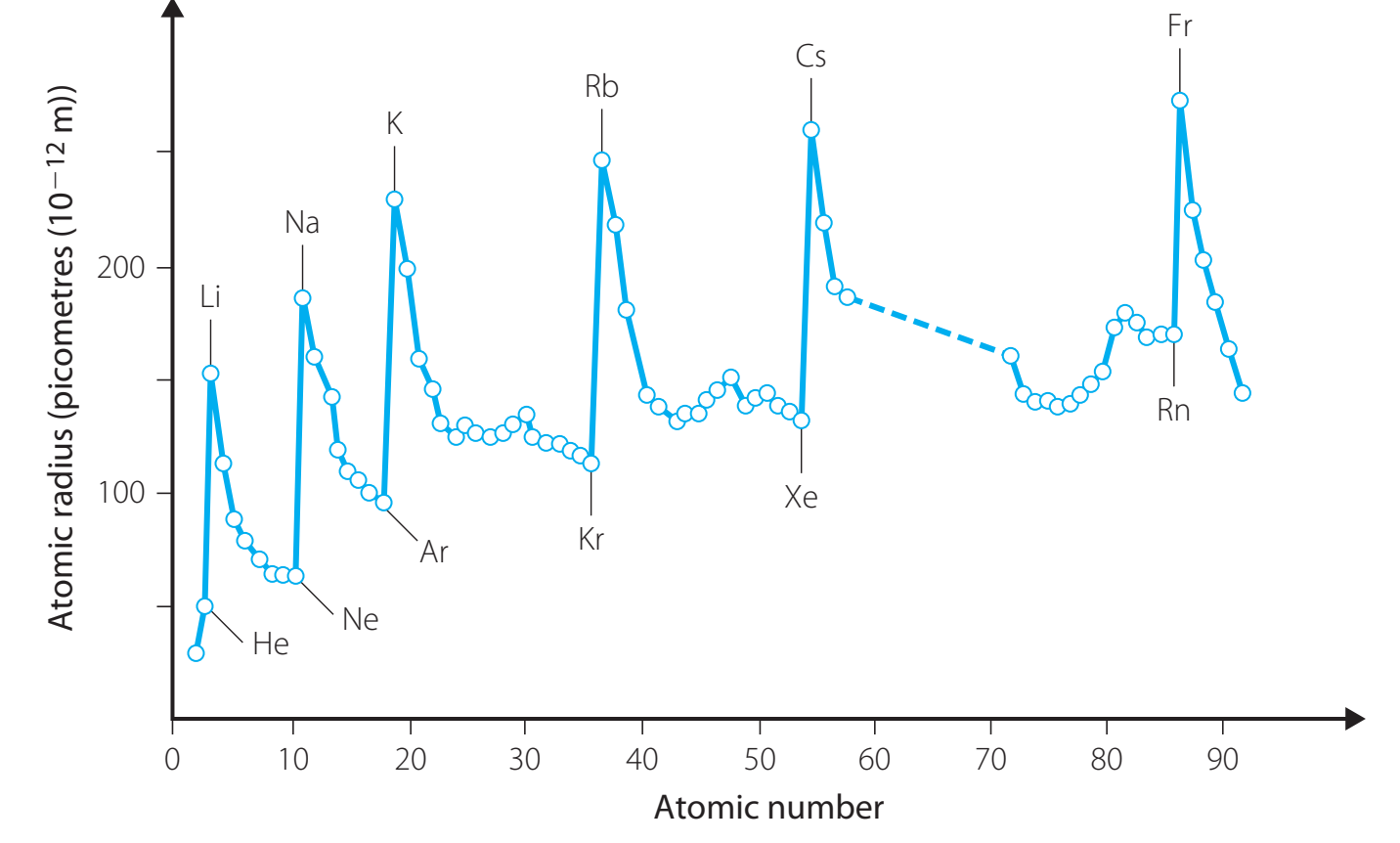
Factors Affecting Electronegativity
-
Periodic Trends:
- Increase Across Period: Electronegativity typically increases across a period (from left to right) due to an increase in effective nuclear charge.
- Decrease Down Group: Electronegativity often decreases down a group (from top to bottom), as atomic size enlarges and the screening effect from inner electrons diminishes nuclear attraction.
-
Atomic Size:
- Smaller atoms exhibit higher electronegativity since their nuclei more effectively attract bonding electrons.
-
Nuclear Charge:
- An increased nuclear charge implies more protons attracting the electron cloud, raising electronegativity.
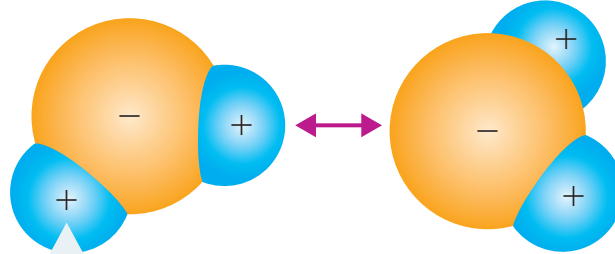
Understanding Bond Polarity
Bond Polarity: Bond polarity arises from the unequal sharing of electrons in covalent bonds due to differences in electronegativity, causing an imbalance in charge distribution.
Dipole Moments: Dipole moments quantify the charge separation in a molecule and are calculated as , where represents the charge and denotes the distance. An increase in either or raises the dipole.
- Example Calculation: For HF, given C and nm, the dipole moment Cm.
Bond Polarity: Occurs when electrons are shared unequally, leading to charge imbalances.
Dipole Moments: Indicate molecular charge separation, calculated via .
Ionic vs. Covalent Bonds
-
Ionic Bonds:
- Form through a significant electronegativity difference (>1.5), common in compounds formed between metals and non-metals.
-
Covalent Bonds:
- Arise with smaller electronegativity differences, implying electron sharing between atoms.
Examples:
- NaCl (Sodium Chloride): Exhibits ionic bonding due to a substantial electronegativity difference.
- Cl₂ (Chlorine molecule): Demonstrates covalent bonding, involving a minor electronegativity difference.
- Summary of Bond Identification: Ionic if > 1.5; Covalent if < 1.5.
Degree of Polarity
Polarity: Polarity involves the uneven distribution of electrical charge within a molecule. It is crucial in determining physical attributes like solubility, boiling points, and conductivity.
- Influence of Polarity:
- Increased polarity enhances intermolecular forces, resulting in elevated boiling points and solubility.
- Note how increased polarity strengthens molecular forces, influencing boiling and melting points.
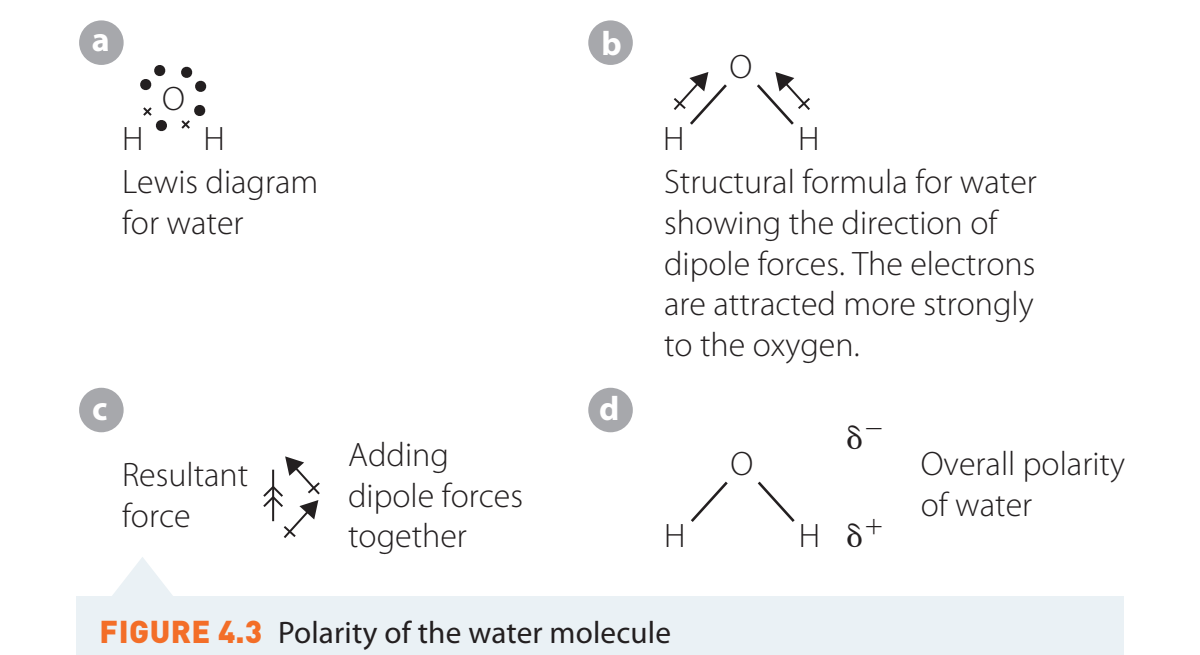
Case Studies and Molecular Polarity
Water (H₂O)
- Structure: Bent shape, creating a net dipole moment due to uneven charge distribution.
- Polarity: A highly polar molecule.
Example:
Carbon Dioxide (CO₂)
- Structure: Linear shape, leading to dipole cancellation.
- Polarity: Non-polar owing to its symmetrical configuration.
Example:
Hydrogen Fluoride (HF)
- Structure: Single polar covalent bond with significant electronegativity difference.
- Polarity: Highly polar due to fluorine's strong electronegativity.
Example:

Speciation Methods
Speciation: The distribution of a substance into various chemical forms within a solution or compound. It is crucial for analysing chemical behaviours.
Spectroscopy Tools
- Infrared (IR) Spectroscopy:
- Identifies functional groups and is widely applied in quality control to ensure product consistency.
- Nuclear Magnetic Resonance (NMR) Spectroscopy:
- Offers insights into molecular structure, extensively used in the pharmaceutical industry for drug research.
- Mass Spectrometry (MS):
- Determines molecular mass and assists in identifying unknown substances, particularly useful in forensic analysis.
Chromatography
- Purpose and Application:
- Critical for separating compounds based on polarity, instrumental in detecting chlorinated hydrocarbons in water.
Explanation with Diagrams
Practical Connections
- Use the diagrams to correlate abstract principles with real-world applications, such as how salt solutions increase conductivity when dissolved in water.
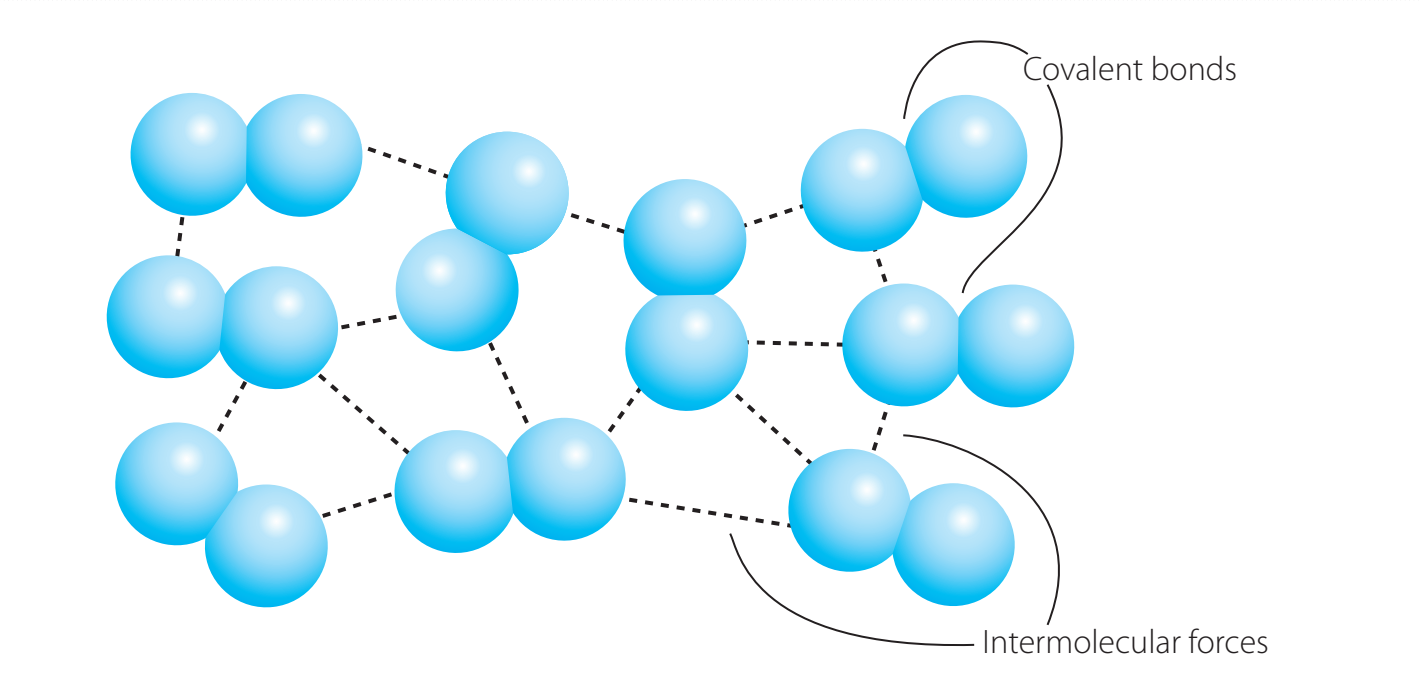
Practice Problems with Solutions
-
Calculate Electronegativity Differences:
- Example: Given Fluorine (F = 3.98) and Hydrogen (H = 2.20), the difference: , indicating a polar covalent bond.
-
Determine Bond Types:
- Solution: If we have a bond between oxygen (3.44) and carbon (2.55), the difference is , which indicates a polar covalent bond.
- Solution: For a bond between sodium (0.93) and chlorine (3.16), the difference is , indicating an ionic bond.
Quick Calculation Tips:
- Differences > 1.5 suggest ionic bonds.
- Differences < 1.5 usually indicate covalent bonds.
- Molecular Polarity Examples:
-
Problem: Determine the polarity of CH₄ (methane).
-
Solution: Carbon (2.55) and hydrogen (2.20) have a small electronegativity difference (0.35). Additionally, CH₄ has a tetrahedral geometry with symmetrical arrangement of bonds. Therefore, the individual bond dipoles cancel each other out, making methane non-polar.
-
Problem: Determine the polarity of NH₃ (ammonia).
-
Solution: Nitrogen (3.04) and hydrogen (2.20) have an electronegativity difference of 0.84, making the N-H bonds polar. NH₃ has a trigonal pyramidal geometry with a lone pair on nitrogen, creating an asymmetrical charge distribution. Therefore, ammonia is a polar molecule.
-
Problem: Determine the polarity of OF₂ (oxygen difluoride).
-
Solution: Oxygen (3.44) and fluorine (3.98) have an electronegativity difference of 0.54, making the O-F bonds polar. OF₂ has a bent geometry due to the two lone pairs on oxygen. The bond dipoles do not cancel, resulting in a polar molecule.
-
Real-World Applications
Real-World Context:
-
Conductivity Example: When table salt (NaCl) is dissolved in water, it forms Na⁺ and Cl⁻ ions that allow the solution to conduct electricity well. In contrast, a sugar solution does not conduct electricity as effectively because sugar molecules do not form ions in solution but remain as whole molecules.
-
Solubility Example: The separation of oil and water in salad dressing illustrates how differences in polarity affect solubility. Water molecules are polar and interact strongly with each other through hydrogen bonding, while oil molecules are non-polar. Since "like dissolves like," the polar water molecules prefer to associate with other polar molecules, excluding the non-polar oil and creating distinct layers.
Glossary of Key Terms:
- Polarity: Uneven distribution of electrical charge.
- Speciation: Various chemical forms in solutions.
- Intermolecular Forces: Forces between molecules that affect properties.
500K+ Students Use These Powerful Tools to Master Electronegativity and Bond Polarity For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
197 flashcards
Flashcards on Electronegativity and Bond Polarity
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Electronegativity and Bond Polarity
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes8 questions
Exam questions on Electronegativity and Bond Polarity
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Electronegativity and Bond Polarity
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Electronegativity and Bond Polarity
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Electronegativity and Bond Polarity you should explore
Discover More Revision Notes Related to Electronegativity and Bond Polarity to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Intermolecular Forces and Allotropy
Carbon's Different Forms
340+ studying
189KViews