Photo AI
Last Updated Sep 24, 2025
Electronegativity Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Electronegativity quickly and effectively.
358+ students studying
Electronegativity
Introduction
- Electronegativity: The tendency of an atom within a molecule to attract electrons towards itself. It plays a vital role in predicting chemical reactions and identifying types of bonds.
- Relevance: Comprehending these patterns aids in accurately predicting the behaviour of elements and chemical reactions.
What is Electronegativity?
- Electronegativity: The tendency of an atom to attract a shared pair of electrons.
- Importance: This concept is crucial for grasping chemical bonding and the properties of compounds.
- Dimensionless Quantity:
- It is defined without units and is usually measured on the Pauling scale.
Different definitions and models of electronegativity may not be universally accepted or applied.
Periodic Trends in Electronegativity
- General Trends:
- Increases across periods from left to right, due to the higher nuclear charge.
- Decreases down groups as additional electron shells provide shielding, reducing the nuclear attraction.
Historical Development
- Key Contributor: Linus Pauling made notable contributions to the understanding of electronegativity.
- Progression: The concept evolved from qualitative ideas to quantitative scales.
Different Electronegativity Scales
- Scales Overview:
- Pauling Scale: Most widely used and recognised.
- Mulliken Scale: Considers both electron affinity and ionisation energy.
- Allred-Rochow Scale: Based on electrostatic forces between nuclei and electrons.
While the Pauling scale is predominant, alternative scales provide valuable insights in specific scientific contexts.
Visual Representation
Bond Classification by Electronegativity
- Electronegativity Differences and Bond Types:
- Ionic Bonds: Electronegativity difference greater than 1.7.
- Example: Sodium chloride (NaCl).
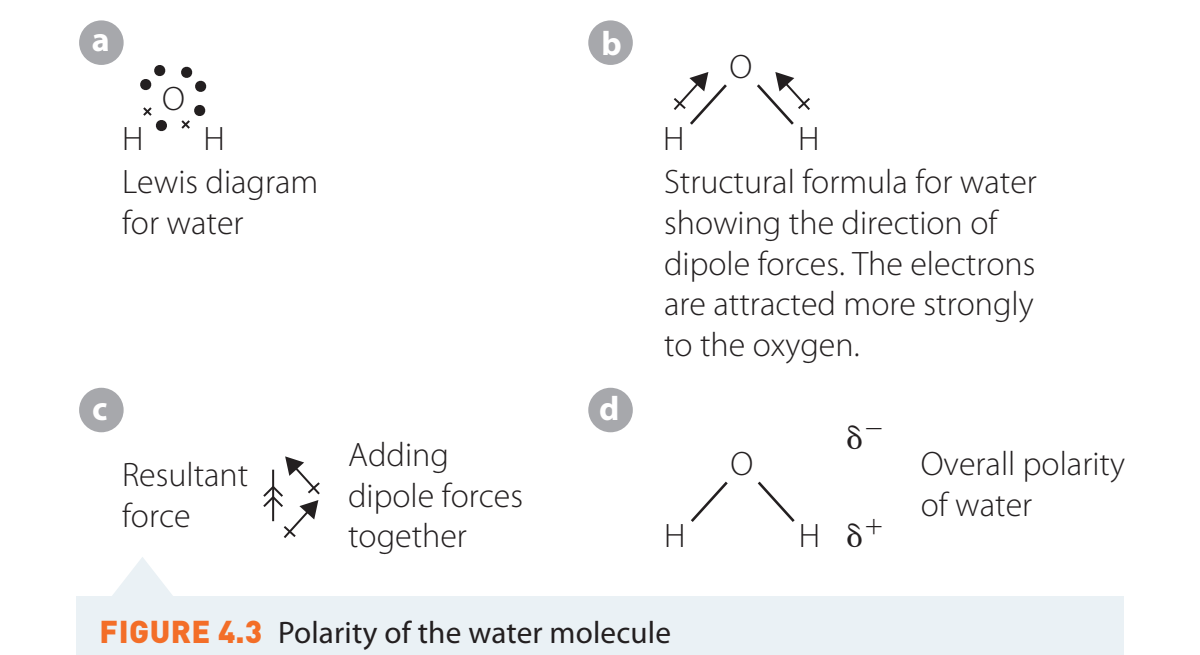
- Polar Covalent Bonds: Electronegativity differences between 0.5 and 1.7.
- Example: Water (H₂O).
- Non-Polar Covalent Bonds: Electronegativity difference less than 0.5.
- Example: Chlorine gas (Cl₂).
- Ionic Bonds: Electronegativity difference greater than 1.7.
Metal Reactivity and Electronegativity
- Inverse Relationship: Metals with low electronegativity are typically highly reactive, especially within the alkali metal group.
- Reactive Metals:
- Lithium (0.98), Sodium (0.93), Potassium (0.82) are notably reactive.
Common Misconceptions
- Noble Gases: Typically excluded from electronegativity considerations due to their full valence electron shells.
- Visualising Trends: Use diagrams to clarify misunderstandings.
Exam Tips
- Understanding Trends: Remember that electronegativity increases across a period and decreases down a group.
- Utilise Visuals: Charts and diagrams are invaluable study aids.
- Practice Problems: Apply scenarios to comprehend how electronegativity influences bonding.
Example Questions
-
Determine the type of bond that forms between lithium (Li) and fluorine (F). Solution: Electronegativity of Li = 0.98, F = 3.98. Difference = 3.98 - 0.98 = 3.0, indicating an ionic bond.
-
Predict the bond type in carbon dioxide (CO₂). Solution: Electronegativity of C = 2.55, O = 3.44. Difference = 3.44 - 2.55 = 0.89, indicating a polar covalent bond.
Worked Examples
- NaCl: Calculate , indicating an ionic bond.
- H₂O: Calculate , indicating a polar covalent bond.
500K+ Students Use These Powerful Tools to Master Electronegativity For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
201 flashcards
Flashcards on Electronegativity
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards19 quizzes
Quizzes on Electronegativity
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Electronegativity
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Electronegativity
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Electronegativity
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Electronegativity you should explore
Discover More Revision Notes Related to Electronegativity to Deepen Your Understanding and Improve Your Mastery
