Photo AI
Last Updated Sep 24, 2025
Melting Point Trends Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Melting Point Trends quickly and effectively.
460+ students studying
Melting Point Trends
Introduction
Understanding the melting points of elements is essential for predicting their behaviour and determining their suitability for various applications. The melting point provides insights into the stability of materials under different temperature conditions. By comprehending the principles of atomic bonding, scientists can make well-informed decisions in both industrial and scientific contexts.
Introduction to Periodicity
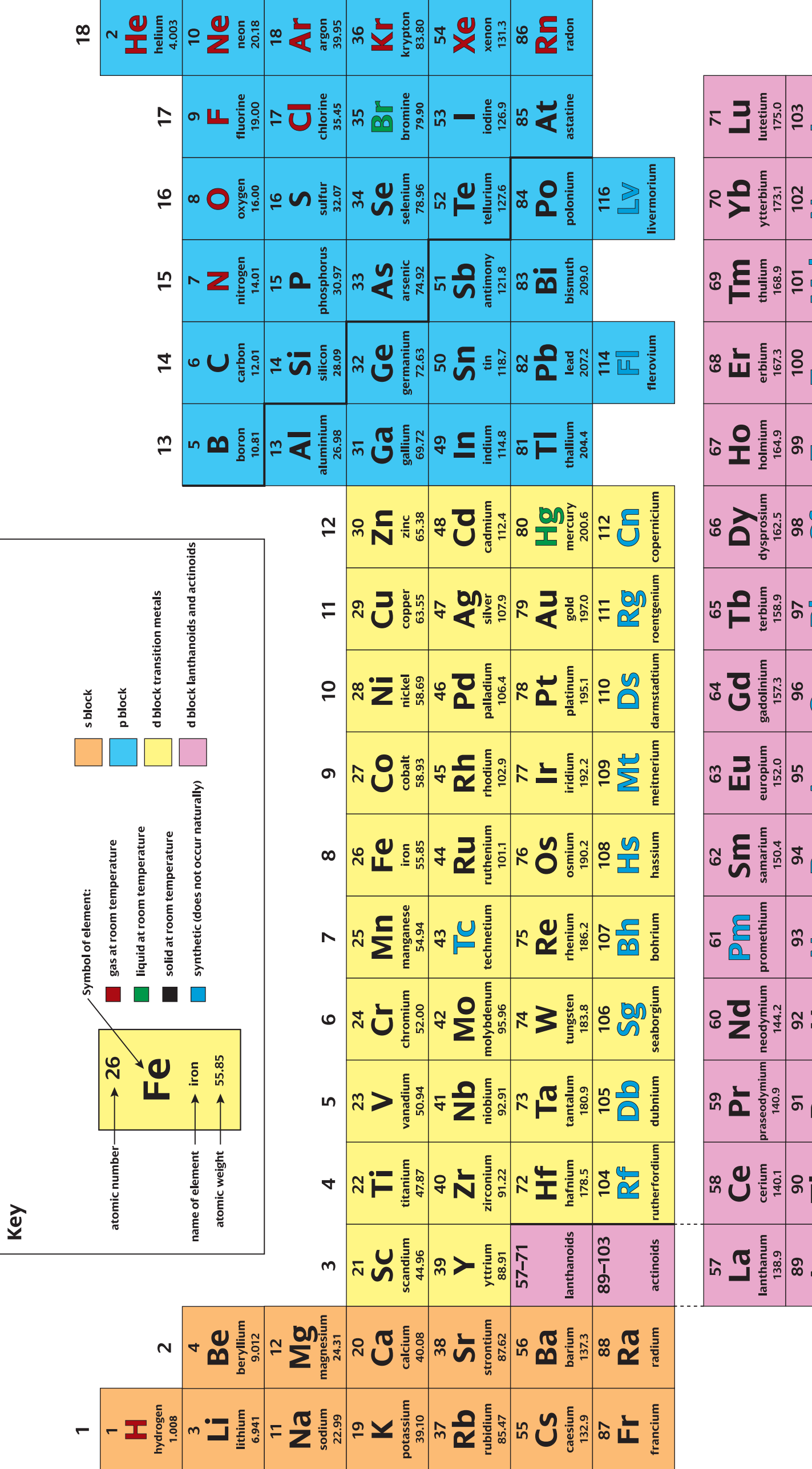
Overview of Periodic Table Organisation
- Periods: These are the rows on the periodic table.
- Groups: These are the columns, also referred to as families.
- Blocks: The periodic table's regions are divided as follows:
- s-block: Includes Groups 1 and 2 as well as Helium.
- p-block: Comprises Groups 13 to 18.
- d-block: Encompasses transition metals, specifically Groups 3 to 12.
- f-block: Covers Lanthanides and Actinides.
- Structure: Organised by increasing atomic number and electron configuration.
History and Rationale Behind the Periodic Table
- Dmitri Mendeleev: Developed the first periodic table in 1869.
- Key Insight: He organised elements by atomic mass and predicted the existence of undiscovered elements.
- Modern Table: Currently arranged by atomic number due to Moseley's 1913 research.
- Rationale: It demonstrates periodic trends in reactivity and properties.

Milestones of the Periodic Table Development:
- 1869 - Mendeleev introduces his periodic table.
- 1913 - Moseley organises it by atomic number.
- Modern layout reflects electron configurations.
Electron Configuration and Element Position
- Position Determination: An element's position is linked to its electron configuration.
- Valence Electrons: The outer shell electrons that are critical to chemical behaviour.
Definitions:
- Valence Electrons: Electrons in the outermost shell that influence chemical reactions.
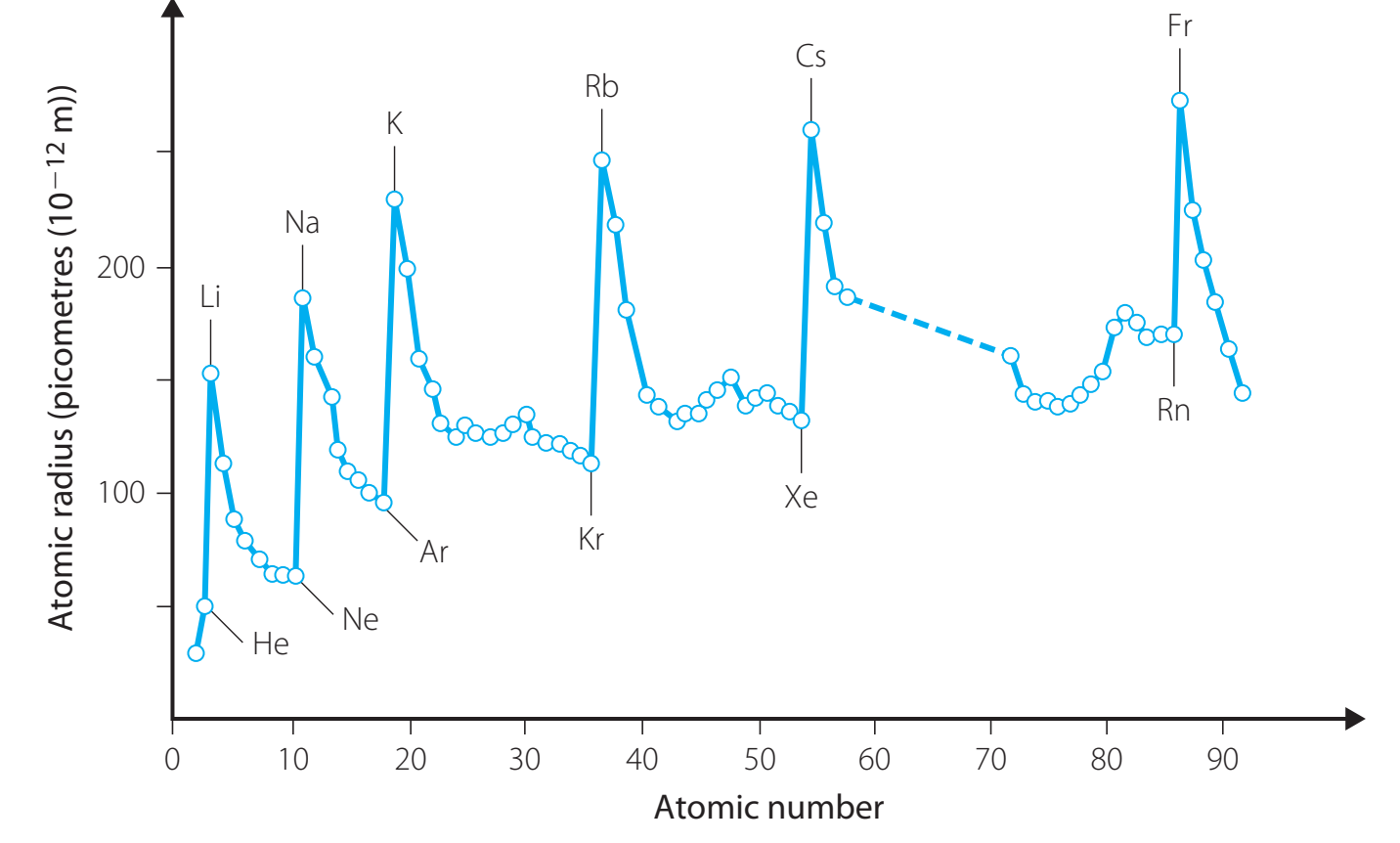
- Atomic Radius: The distance from the nucleus to the boundary of the electron cloud.
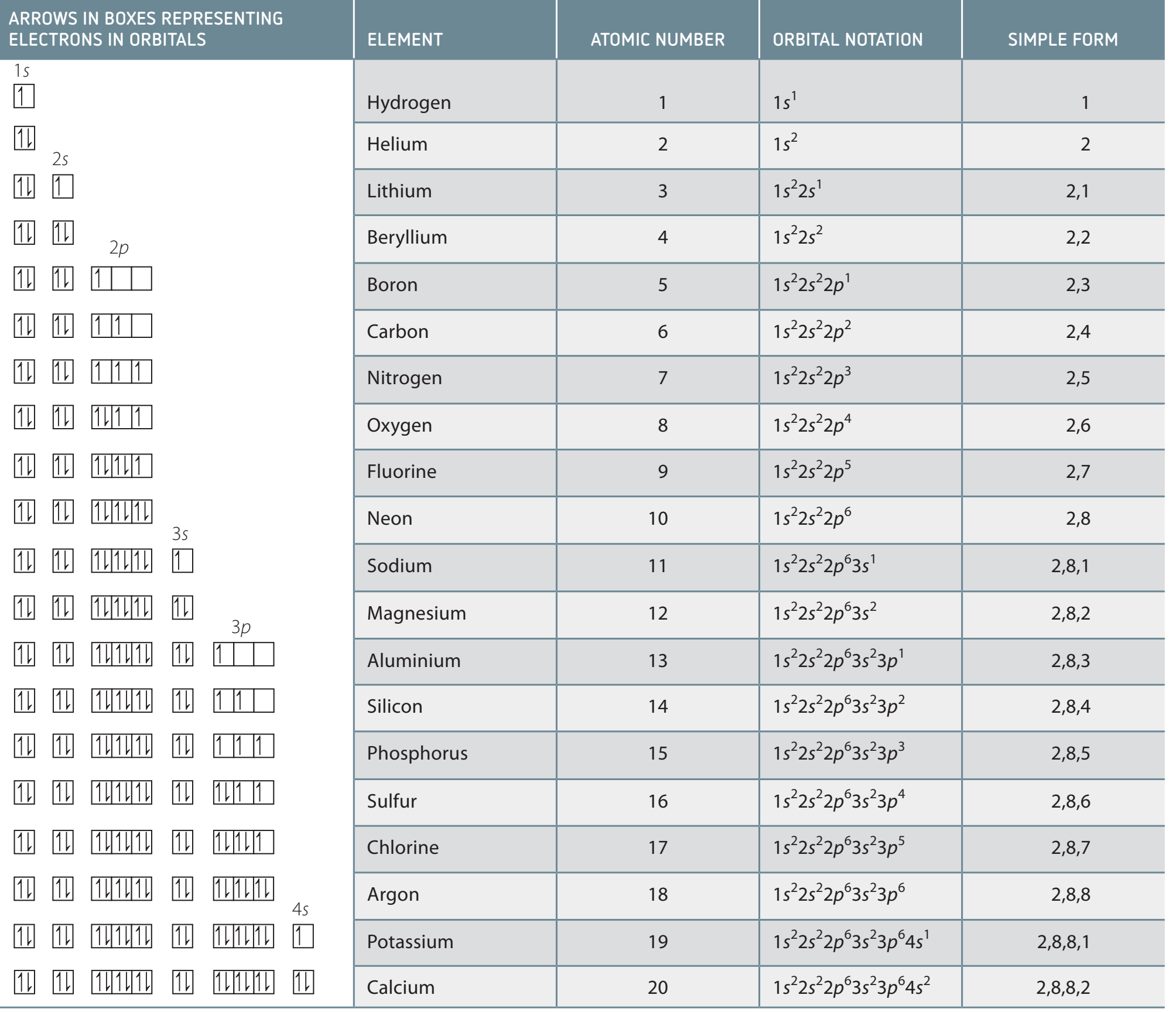
Trends Across Groups and Periods
- Atomic Radius:
- Decreases across a period.
- Increases down a group.
- Ionisation Energy:
- Increases across a period.
- Decreases down a group.
- Electron Affinity:
- Becomes more negative across a period.
Summary of Key Trends:
- Atomic radius decreases moving to the right, and increases moving down a group.
- Ionisation energy generally increases to the right and decreases down.
- Electron affinity becomes more negative to the right.
Defining Melting Point
What is Melting Point?
Melting Point temperature at which a solid transitions into a liquid.
- Physical Process: At this temperature, particles in a solid overcome their fixed positions and begin to move freely, becoming a liquid.
- Significance: Indicates material properties, such as composition and purity levels. It is vital in material science and industrial settings for ensuring quality and determining suitability for various applications.
Intermolecular Forces and Melting Point
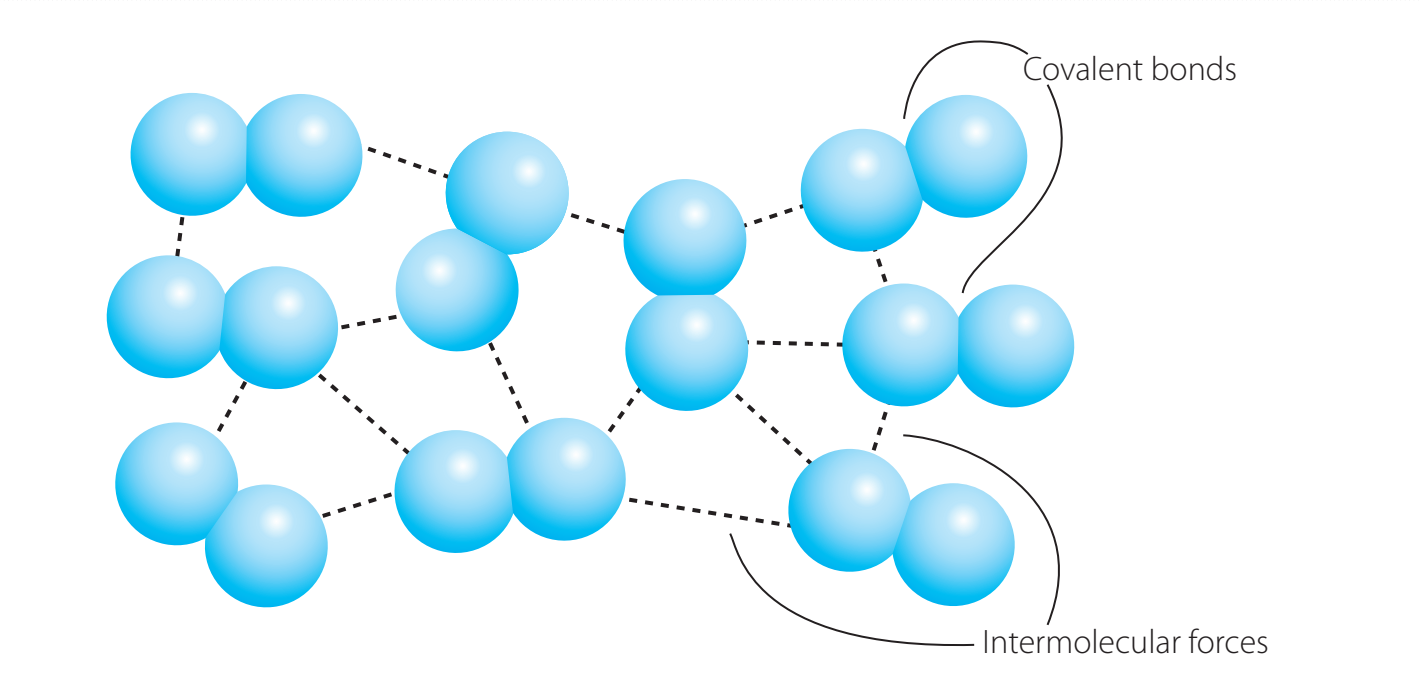
- Determining Melting Points: The type and strength of intermolecular forces significantly impact melting points.
- Van der Waals Forces: Weak interactions, resulting in lower melting points.
- Example: Noble gases like Argon.
- Hydrogen Bonds: Moderate strength, leading to higher melting points.
- Example: Water, which requires more energy to disrupt the bonds.
- Covalent Bonds: Strong bonding, leading to exceptionally high melting points.
- Example: Diamond, which demands significant energy to alter its structure.
- Van der Waals Forces: Weak interactions, resulting in lower melting points.
Comparing Melting Points of Various Substances
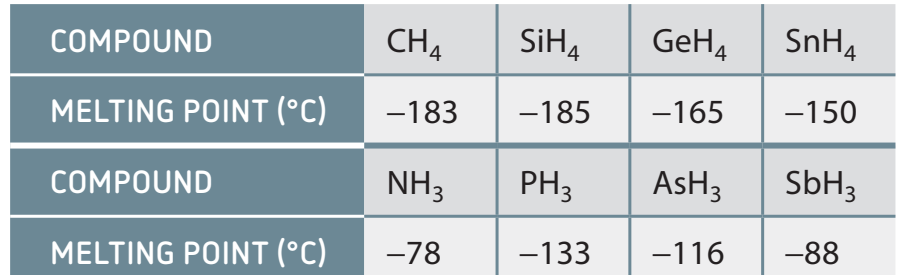
- Metals: Typically have high melting points due to metallic bonding.
- Example: Iron.
- Ionic Compounds: Generally exhibit high melting points as a result of ionic bonding.
- Example: Sodium chloride (table salt).
- Molecular Compounds: May display varying, often lower, melting points depending on their bonding types.
- Example: Carbon dioxide (dry ice), which sublimates rather than melts at atmospheric pressure.
Overview of Trends in Melting Points
- Melting Point: The temperature where solid becomes liquid—key for predicting elemental behaviour.
Understanding trends in melting points aids in forecasting the behaviours of unknown elements and refining chemical synthesis processes.
- Key Takeaways:
- Aids in predicting unknown elements' behaviours.
- Essential in enhancing chemical synthesis.
Detailed Periodic Trends
Melting Point Variation Across Periods
- General Trend:
- Period 2:
- • Increasing: Li, Be
- ◦ Peak: B, C
- • Decreasing: N, O, F, Ne
- Period 3:
- • Increasing: Na, Mg
- ◦ Peak: Al, Si
- • Decreasing: P, S, Cl, Ar
- Transition Metals: Display variability due to d-orbital influence.
- Period 2:
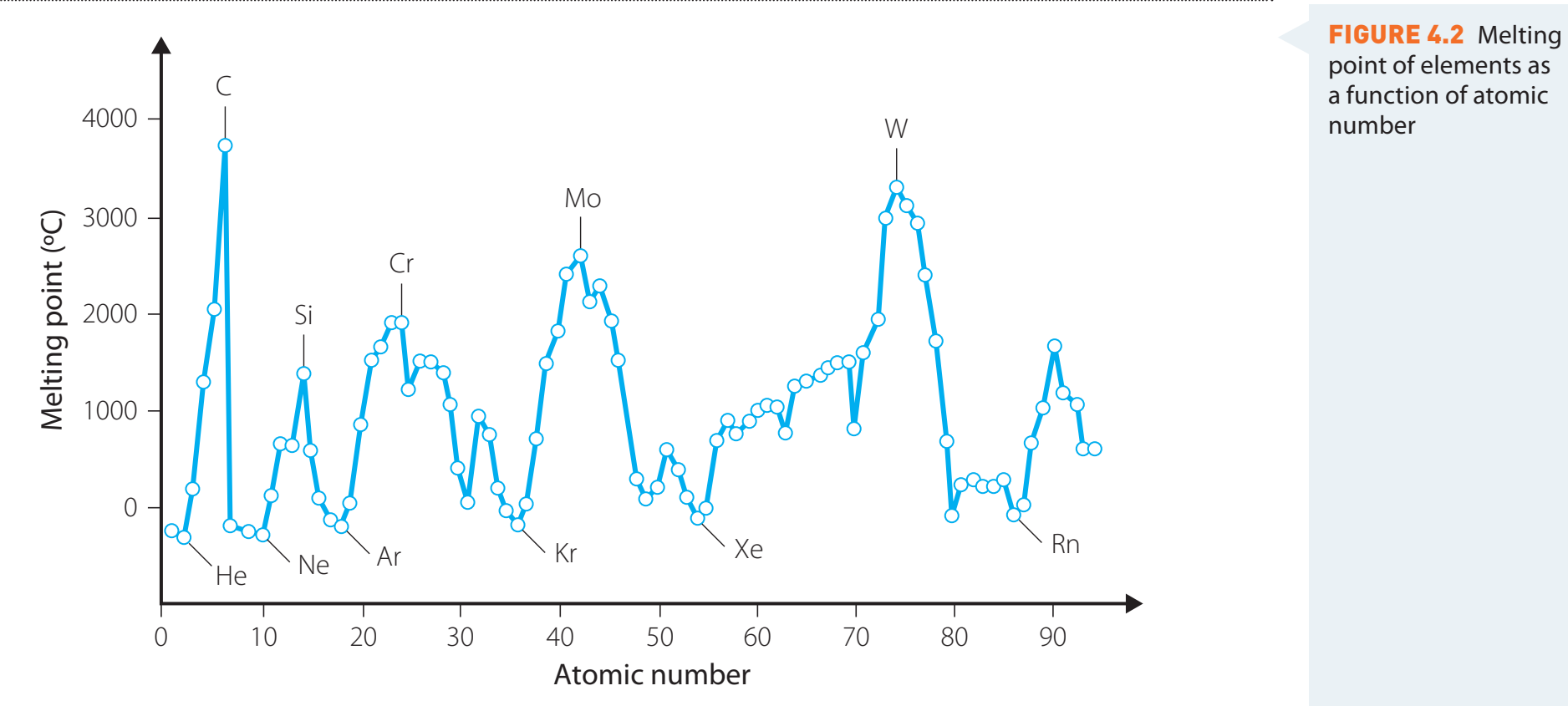
Observe strong covalent interactions peaking at Group 14.
Group Trends
Trends in Alkali Metals and Halogens
-
Alkali Metals:
- Trend: Melting points decrease down the group. Increased atomic size weakens metallic bonds.
-
Halogens:
- Trend: Decreased melting points are seen due to the larger atomic size weakening intermolecular forces.
Analysis of Trends
Analysing Periodic and Group Trends
- Correlation With Atomic Properties:
-
chatImportant
Increased atomic size weakens bonds, reducing melting points; electronegativity strengthens bonds, increasing melting points.
-
Impact of Subatomic Features
- Role of Electron Configuration:
- Electron configurations significantly influence bonding within periods and groups.

Introduction to Factors Affecting Melting Point
Understanding the factors affecting melting points is vital for predicting material behaviour under varying conditions. These factors encompass atomic size, charge density, bonding types, intermolecular forces, and crystal structures.
Subsection 1: Atomic Size & Charge
-
Atomic Size Effect: Smaller atomic sizes tend to form stronger bonds, thus resulting in higher melting points.
- Trends & Examples: Across a period, atomic size decreases, causing an increase in melting points. Elements like oxygen and fluorine follow this trend.
infoNoteAtomic Size: The distance from the nucleus to the electron cloud boundary, a key aspect in understanding bond strength.

- Charge Density Influence: A pivotal determinant of bond strength is charge density. Higher charge densities bolster bonds, leading to increased melting points.
- Examples: Calcium ions () have higher charge densities compared to potassium ions ().
Charge Density: The charge per unit volume, a crucial factor in determining the strength of atomic bonds and melting points.
Subsection 2: Bonding Types
-
Metallic Bonding:
- Examples: Iron (Fe), Copper (Cu)
- Characteristics: Delocalised electrons result in high melting points.
-
Ionic Bonding:
- Examples: Sodium chloride (NaCl), Magnesium oxide (MgO)
- Characteristics: Strong electrostatic attractions contribute to high melting points.
-
Covalent Network Bonding:
- Examples: Diamond (C), Quartz (SiO)
- Characteristics: Extensive atomic networks confer very high melting points.
-
Molecular Bonding:
- Examples: Water (HO), Carbon Dioxide (CO)
- Characteristics: Weaker forces result in lower melting points.
Subsection 3: Intermolecular Forces
-
Van der Waals Forces:
- Present in: Noble gases such as Helium (He), Neon (Ne)
- Role: Responsible for low melting points due to their weak interactions.
-
Hydrogen Bonds:
- Present in: Water (HO), Ammonia (NH)
- Role: Play a significant role in raising melting points due to their strength.
-
Dipole-Dipole Interactions:
- Present in: Hydrogen chloride (HCl)
- Role: Lead to intermediate melting points.
Intermolecular Forces: These forces act between molecules and are crucial in determining melting and boiling points.
Subsection 4: Crystal Structure & Packing
-
Crystal Influence: The atomic arrangement in a crystal lattice dramatically affects melting point stability.
-
Packing Efficiency: Denser arrangements generally lead to higher melting points due to enhanced stability.

High Melting Point Examples
Definition and Importance
-
High Melting Points: These indicate stability at high temperatures as a result of strong atomic bonds.
-
Tungsten (W):
- Properties:
- Melting Point: 3422°C
- Atomic Mass: 183.84
- Bonding Type: Metallic
- Definition of Metallic Bonding:
- Metallic bonds feature shared electrons within tightly packed structures, strengthened by d-orbital overlaps.
- Industrial Use:
- Crucial for high-temperature applications such as light bulb filaments.
- Essential for high thermal endurance.
- Properties:
-
Diamond (C):
- Properties:
- Melting Point: ~3550°C
- Atomic Mass: 12.01
- Bonding Type: Covalent Network
- Definition of Covalent Network:
- Covalent networks have strong directional bonds in a 3D framework, imparting high stability.
- Utility:
- Ideal for tools that resist heat and friction.
- Properties:

Low Melting Point Examples
Definition and Importance
-
Low Melting Points: Facilitate state changes due to weak forces.
-
Mercury (Hg):
- Properties:
- Melting Point: -38.83°C
- Atomic Mass: 200.59
- Bonding Type: Metallic
- Role of Bonding and Characteristics:
- Liquid at room temperature due to weak metallic bonds.
- Utility:
- Commonly used in thermometers.
- Enables precise temperature measurement.
- Properties:
-
Xenon (Xe):
- Properties:
- Melting Point: -111.79°C
- Atomic Mass: 131.29
- Bonding Type: Van der Waals
- Explanation of Van der Waals:
- Van der Waals forces are relatively weak, leading to low melting points.
- Properties:


Comparative Analysis Table
| Element | Melting Point (°C) | Atomic Mass | Bonding Type | Bonding Influence & Application Relevance |
|---|---|---|---|---|
| Tungsten | 3422 | 183.84 | Metallic | Strong d-orbital overlaps. Used in high-temp tasks. |
| Diamond | ~3550 | 12.01 | Covalent Network | Rigid 3D structure. Suitable for cutting tools. |
| Mercury | -38.83 | 200.59 | Metallic | Weak bonds. Liquid state for measurements. |
| Xenon | -111.79 | 131.29 | Van der Waals | Weak forces. Limited practical use. |
Industrial Uses and Implications
Understanding melting points is crucial in material selection across various sectors, particularly in metallurgy and related scientific fields.
500K+ Students Use These Powerful Tools to Master Melting Point Trends For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
201 flashcards
Flashcards on Melting Point Trends
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards19 quizzes
Quizzes on Melting Point Trends
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Melting Point Trends
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Melting Point Trends
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Melting Point Trends
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Melting Point Trends you should explore
Discover More Revision Notes Related to Melting Point Trends to Deepen Your Understanding and Improve Your Mastery
