Photo AI
Last Updated Sep 24, 2025
Periodic Trends in Chemistry Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Periodic Trends in Chemistry quickly and effectively.
429+ students studying
Periodic Trends in Chemistry
1. Definition and Importance of Periodicity
- Periodicity: recurrent patterns observed across periods and groups in the periodic table.
- Physical Properties:
- Influence boiling points, melting points, and atomic/ionic sizes.
- Facilitate predictions about density and thermal conductivity.
- Chemical Properties:
- Affect reactivity, electron affinity, and ionisation energy.
- Impact elements' stability and bonding capabilities.
Periodicity is fundamental to chemistry as it allows for the prediction of chemical behaviours and reactions.
2. Arrangement of the Periodic Table
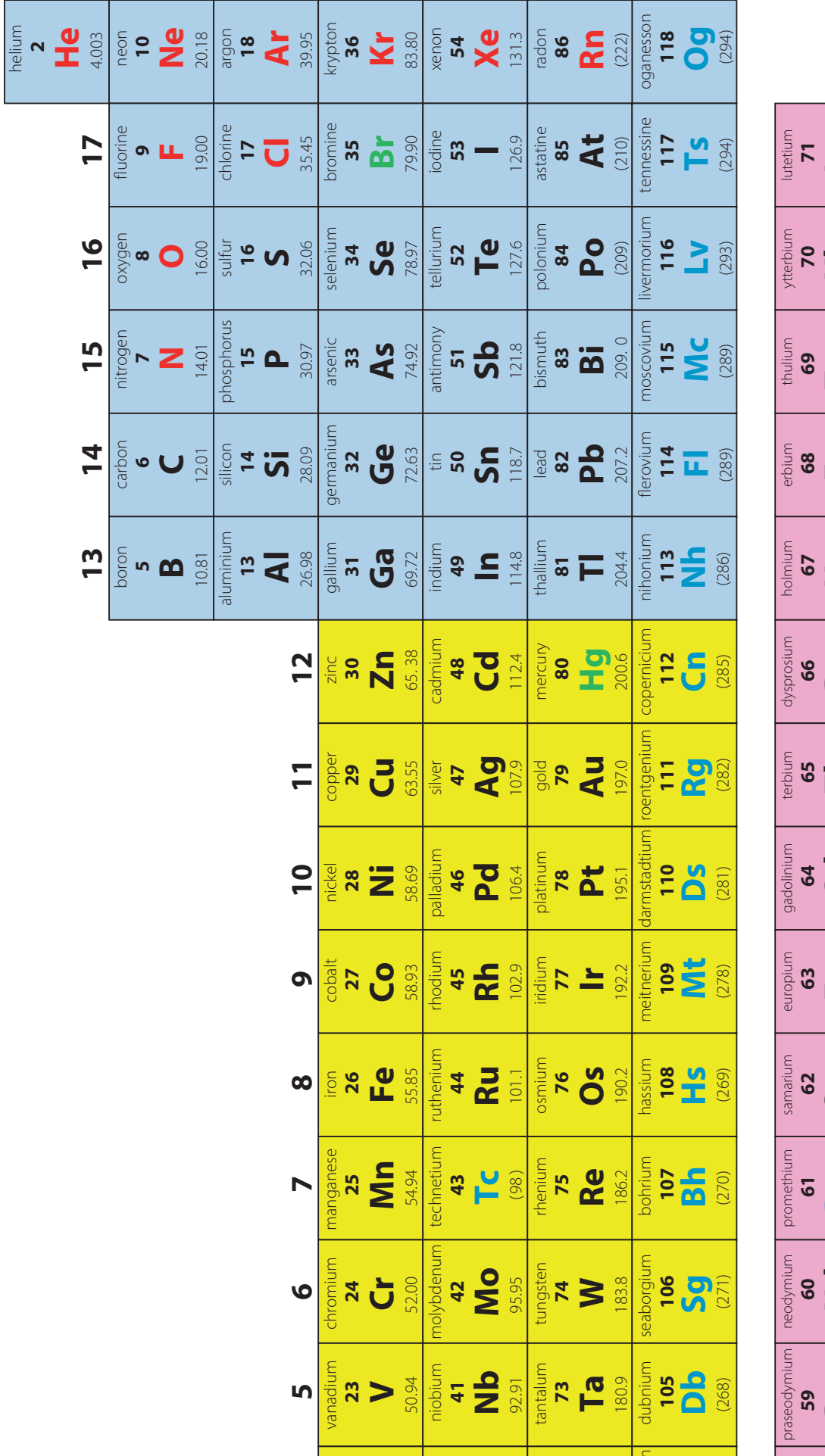
- Overview:
- The table is organised into periods (rows) and groups (columns) to reflect elemental properties.
- This layout aids in predicting trends in properties across various elements.
- Historical Context:
- Dmitri Mendeleev: Created the initial structured arrangement by atomic weight, forming the foundation for the modern periodic framework.
- Did you know? Mendeleev's insight has profoundly shaped our understanding of element prediction.
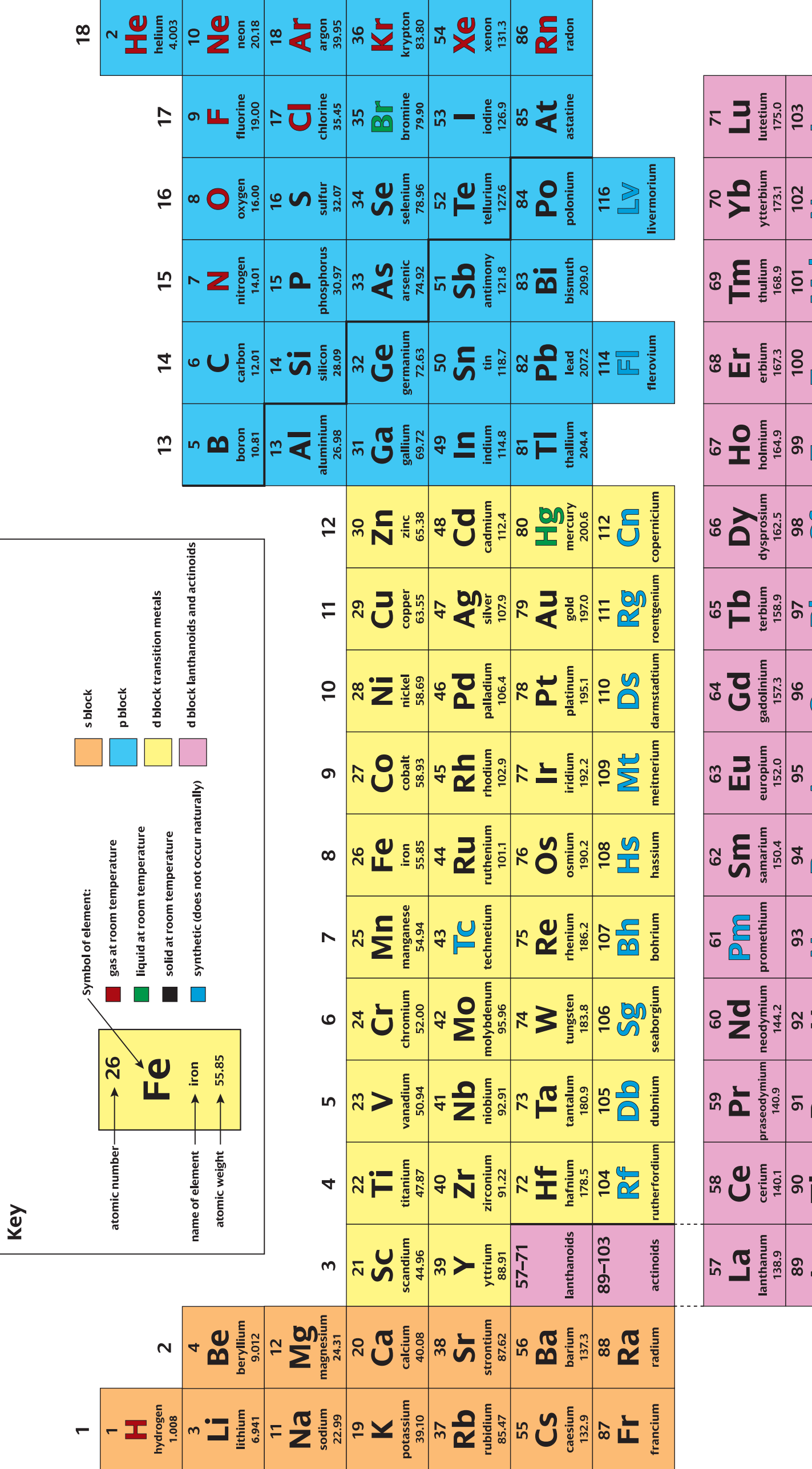
3. Electronic Configurations and State of Matter
- Electronic Configurations:
- Denotes the distribution of electrons around an atom's nucleus.
- Determines an element's state at room temperature.
- Example: Sodium (Na):
- States of Matter:
- Solid: Atoms are closely packed, e.g., Iron (Fe).
- Liquid: Atoms allow fluid movement, e.g., Mercury (Hg).
- Gas: Atoms are widely separated with high energy, e.g., Chlorine (Cl).
Visual Illustrations and Examples
- Diagram: Depicting states of matter for metals, non-metals, and metalloids.
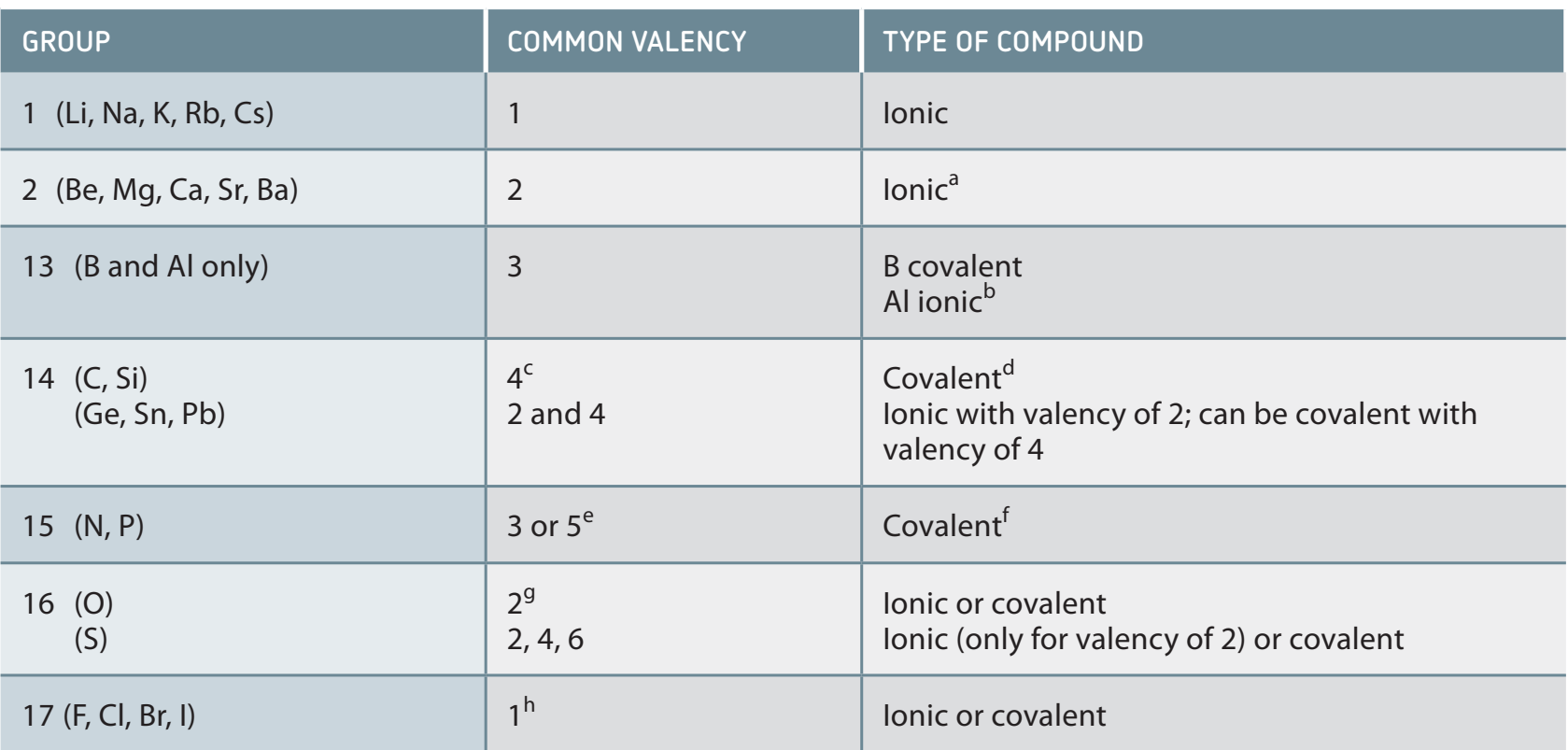
4. Key Trends in Groups and Periods
- Groups:
- Similar chemical properties arise from having the same number of valence electrons.
- Periods:
- Properties consistently evolve with increasing atomic numbers.
- Example:
- Group 2: Notable for acid-base interactions.
- Group 17: Reactivity varies due to changes in electronegativity.
Trends in electronegativity: Increases across a period, decreases down a group, influencing bond formations and reactions.
5. Predicting Chemical Behaviour
- Predictive Power:
- Trends facilitate predictions concerning element reactivity and bonding.
Worked Example
- Comparing Lithium and Potassium Reactivity:
- Both elements are in Group 1 (alkali metals)
- Potassium is below lithium in the group
- As we move down Group 1, reactivity increases because:
- Atomic radius increases (more electron shells)
- Valence electrons are further from the nucleus
- Less energy is required to remove the outer electron
- Therefore, potassium reacts more vigorously with water than lithium
Recognising these trends is crucial for anticipating reactions, essential in laboratory and industrial processes.
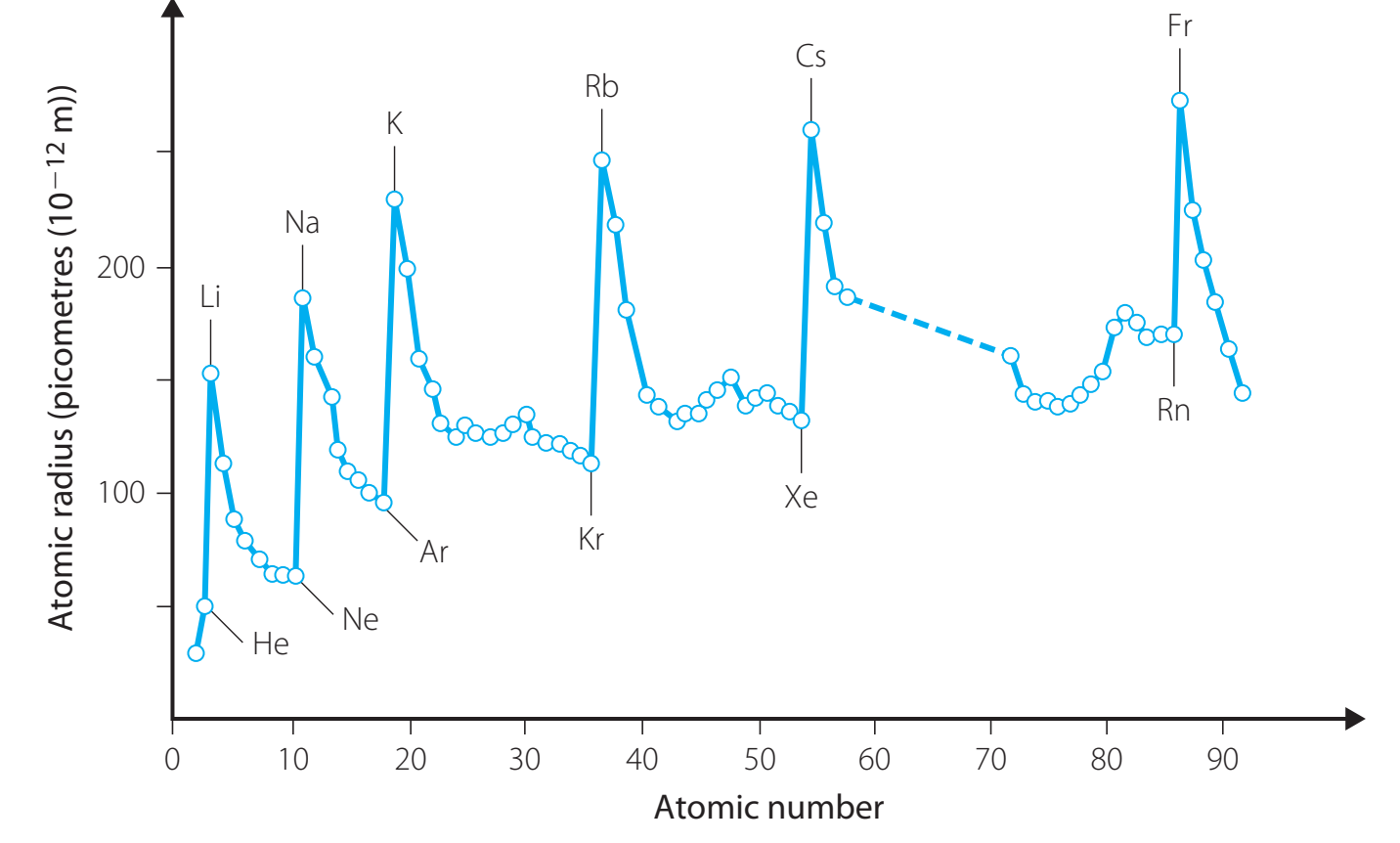
6. Atomic Radii and Ionisation Energy Trends
Atomic Radii
- Trend Across Periods:
- Decreases: Greater nuclear charge draws electrons closer.
- Trend Down Groups:
- Increases: Adding electron shells results in larger atomic size.
Ionisation Energy
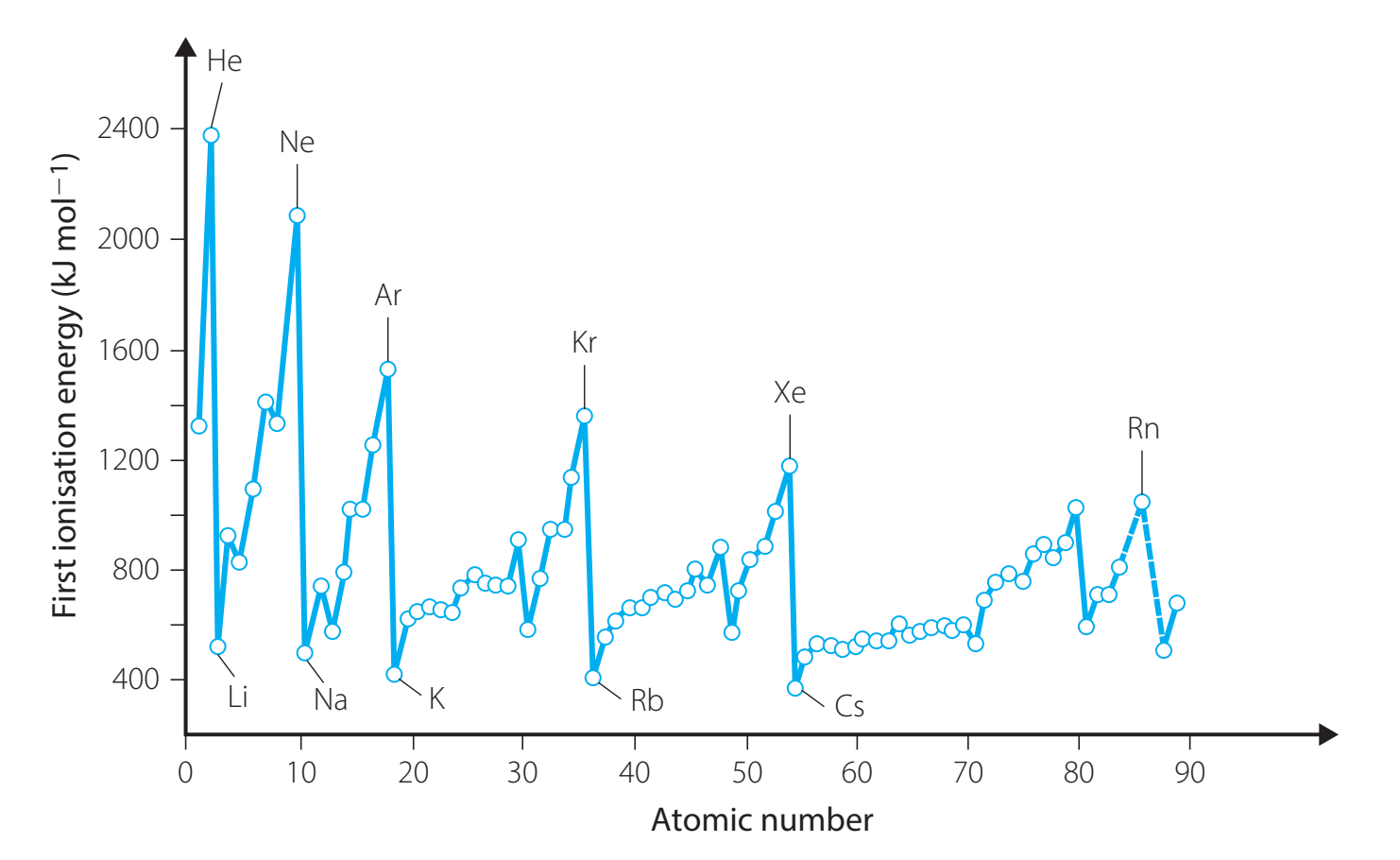
- Across a Period:
- Generally increases.
- Down a Group:
- Generally decreases.
7. Electronegativity
- Definition: The strength of an atom's pull for electrons within a bond.
- Trend Across a Period: Rises from left to right.
- Trend Down a Group: Diminishes.
Understand exceptions such as noble gases with full electron shells, which deviate from typical electronegativity trends.
8. Reactivity with Water
Alkali Metals (Group 1)
- Trend: Reactivity increases down the group.
- Reactions:
- Lithium:
- Sodium:
Impact of reactivity trend: Easier electron release due to larger atomic size as one moves down the group.
Alkaline Earth Metals (Group 2)
- Trend: Less vigorous reactions.
- Reaction Example:
- Calcium:
Safety Considerations
Safety precautions are critical: Rapid hydrogen generation could result in potential hazards.

9. Key Takeaways
- Key Concepts:
- Understanding Periodicity: Essential for forecasting element behaviour and chemical interactions.
- Applications: Crucial in industrial and pharmaceutical innovations.
500K+ Students Use These Powerful Tools to Master Periodic Trends in Chemistry For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
201 flashcards
Flashcards on Periodic Trends in Chemistry
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards19 quizzes
Quizzes on Periodic Trends in Chemistry
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Periodic Trends in Chemistry
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Periodic Trends in Chemistry
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Periodic Trends in Chemistry
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Periodic Trends in Chemistry you should explore
Discover More Revision Notes Related to Periodic Trends in Chemistry to Deepen Your Understanding and Improve Your Mastery
