Photo AI
Last Updated Sep 24, 2025
Ionisation Energy Trends Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Ionisation Energy Trends quickly and effectively.
222+ students studying
Ionisation Energy Trends
Introduction to Ionisation Energy
Ionisation Energy: The energy required to remove an electron from a neutral gaseous atom. It is a fundamental concept in chemistry, essential for understanding the reactivity and stability of elements. Lower ionisation energies indicate higher reactivity, especially among metals.
Definition: Ionisation energy: the energy necessary to remove an electron from a neutral gaseous atom, resulting in the formation of a cation. This measurement is vital for predicting chemical behaviours and interactions.
Fundamental Concepts of Ionisation Energy
First Ionisation Energy
- First ionisation energy: The energy required to remove the first electron from a neutral atom.
- This metric reflects an element's reactivity and stability.
- Each successive electron removal necessitates more energy due to the increased positive charge on the cation.
Definition: First ionisation energy: the energy required to remove the first electron from a neutral atom.
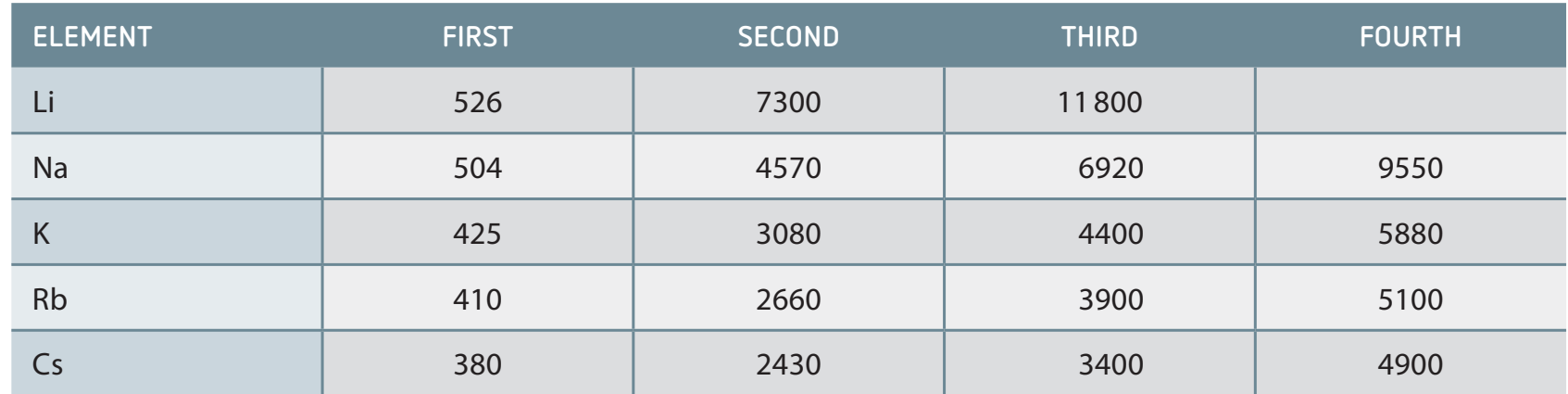
Successive Ionisation Energies
- Ionisation energy increases with each electron removed, as a result of the heightened nuclear charge.
- Example: Sodium (Na):
- First electron removal: Na to Na+
- Additional electrons require increasing energy.
- Attaining a noble gas configuration enhances stability, complicating further ionisation.
Successive ionisation energies showcase the growing stability associated with noble gas configurations.
Formula for Ionisation Energy
Ionisation energy can be determined using the following equation:
- IE = Ionisation Energy
- It represents the difference between the initial energy and the energy post-ionisation.
Numeric Example
- Initial energy of atom () = 500 kJ/mol
- Energy of resulting ion plus electron () = 200 kJ/mol
Calculation:
This shows the energy necessary to remove an electron.
Trends in Ionisation Energy
Across a Period
- Increases from left to right due to an increased nuclear charge, leading to a stronger attraction of electrons.
- Atomic Radius: Decreases across a period, resulting in stronger electron-nucleus interactions.
- Notable Exceptions:
- Be vs. B: The filled s-subshell in Be requires more energy than in B.
- N vs. O: Electron pairing in O decreases the energy compared to N.
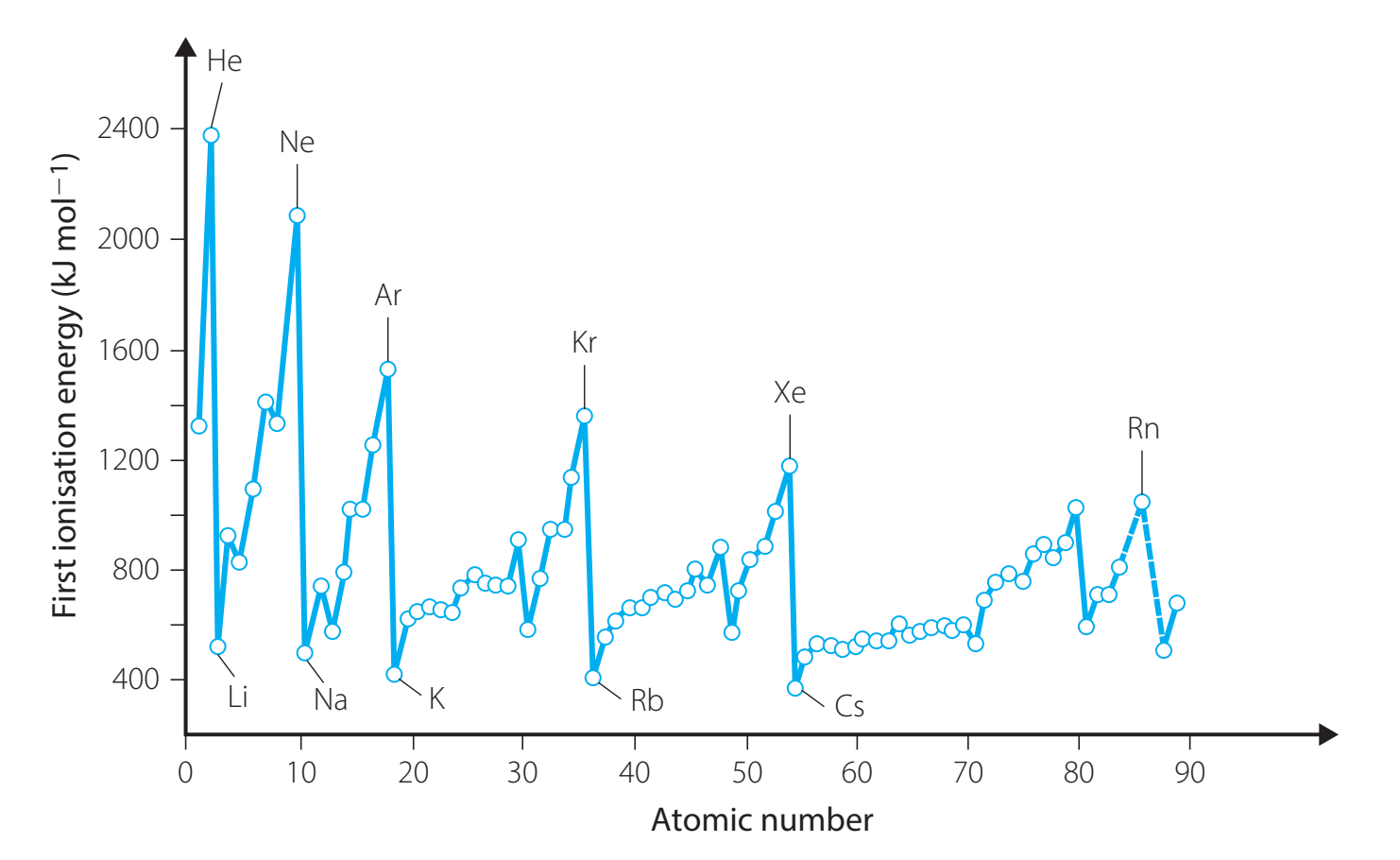
Down a Group
- Decreases down a group because:
- Increasing atomic size places electrons further from the nucleus.
- Enhanced electron shielding: Additional inner electrons reduce the nuclear attraction to outer electrons.
Exceptional Cases
Beryllium vs. Boron
- Despite being to the right of beryllium, boron demonstrates lower ionisation energy.
- The extra electron enters a higher-energy p-subshell.
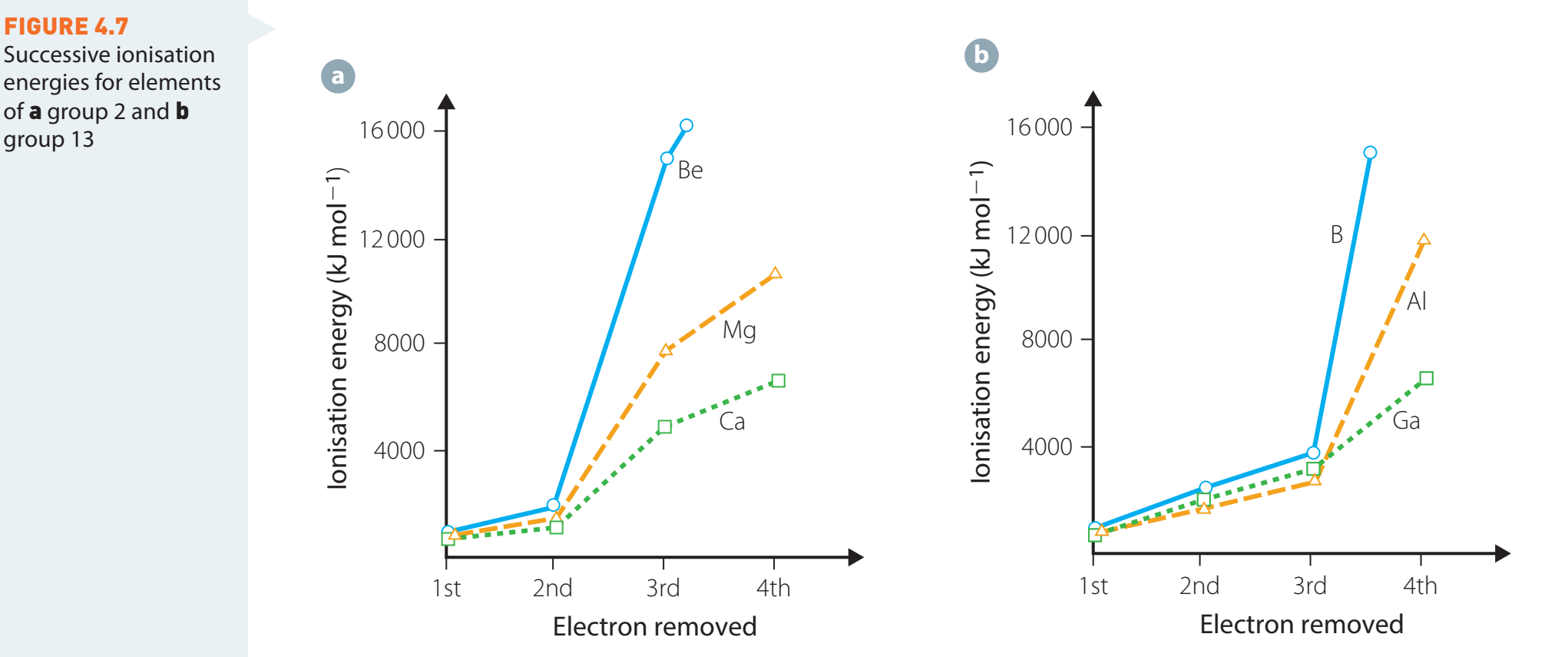
Nitrogen vs. Oxygen
- Oxygen exhibits lower ionisation energy than nitrogen due to electron pairing, which increases repulsion.
Factors Influencing Ionisation Energy
Key Influencing Factors
-
Atomic Size:
- A larger size corresponds with lower ionisation energy.
- Electrons that are more distant from the nucleus are more easily removed.
-
Nuclear Charge:
- A higher nuclear charge increases the energy required to remove an electron.
-
Electron Shielding:
- Inner electrons diminish the nuclear pull on outer electrons.
- Greater shielding results in lower required energy.

Ionisation Energy in Practical Applications
- Industrial Relevance: Metals with low ionisation energy, such as sodium and potassium, are highly reactive.
- Comparison Table:
| Metal | First Ionisation Energy (kJ/mol) | Reactivity Level |
|---|---|---|
| Lithium | 520.2 | Low |
| Sodium | 495.8 | Medium |
| Potassium | 418.8 | High |
Calculation Methods
-
Spectroscopy:
- Emission lines reflect energy levels.
-
Photoelectron Spectroscopy (PES):
- It measures the kinetic energy of electrons after ejection, providing precise ionisation energy assessment.
-
Einstein's Equation:
- Calculate ionisation energy using
Key Study Points
- Trends: Ionisation energy increases across a period and decreases down a group.
- Essential Concepts:
- Atomic Radius affects electron removal ease.
- Nuclear Charge & Shielding are influential factors.
- Exceptions: Observe energy variations due to electron shell configurations.
Practice Problems
Problem 1: Period Trend
- Arrange elements by increasing first ionisation energy within Period 2.
- Solution: Li < Be < B < C < N < O < F < Ne (Elements are arranged from lowest to highest ionisation energy, following the general trend across Period 2)

Problem 2: Group Trend
- Compare ionisation energies in Group 1: Li > Na > K.
- Solution: This ordering is correct. Ionisation energy decreases down Group 1, with Li having the highest and K the lowest due to increasing atomic radius and electron shielding.

Calculation Exercise
- Given frequency Hz, compute ionisation energy using Planck's constant.
- Solution: Using , where J·s
- If (threshold frequency case), then J per atom
- Converting to kJ/mol:
500K+ Students Use These Powerful Tools to Master Ionisation Energy Trends For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
201 flashcards
Flashcards on Ionisation Energy Trends
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards19 quizzes
Quizzes on Ionisation Energy Trends
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes5 questions
Exam questions on Ionisation Energy Trends
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Ionisation Energy Trends
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Ionisation Energy Trends
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Ionisation Energy Trends you should explore
Discover More Revision Notes Related to Ionisation Energy Trends to Deepen Your Understanding and Improve Your Mastery
