Photo AI
Last Updated Sep 24, 2025
Chemistry - Catalysts Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemistry - Catalysts quickly and effectively.
280+ students studying
Chemistry - Catalysts
Introduction to Catalysts
Definition and Role of Catalysts
- Catalysts are agents that enhance the speed of a chemical reaction without undergoing alteration.
- Primary Function: Catalysts decrease the activation energy necessary, thus facilitating quicker reactions.
Catalysts: Agents that enhance the speed of chemical reactions without undergoing alteration.
Types of Catalysts
-
Homogeneous Catalysts:
- Definition: Function within the same phase as the reactants, such as liquid with liquid.
- Example: In salad dressing, vinegar (acetic acid) serves as a catalyst in breaking down oils.
infoNoteFunction within the same phase as reactants (e.g., liquid with liquid).
-
Heterogeneous Catalysts:
- Definition: Operate in a different phase from the reactants, such as solid with liquid.
- Example: Iron is utilised in the Haber process for ammonia synthesis.
infoNoteOperate in a different phase from reactants (e.g., solid with liquid).
Biological Catalysts (Enzymes)
-
Definition and Significance:
- Enzymes are biological catalysts that enhance reactions within living organisms, akin to unlocking specific keys.
- Example: Enzymes in laundry detergents aid in removing stains, while amylase in saliva breaks down carbohydrates.
infoNoteEnzymes: Biological catalysts that accelerate reactions within living organisms.
Industrial Importance of Catalysts
-
Haber Process:
- Catalysts are vital in the production of ammonia, essential for fertilisers. Without catalysts, this process would be considerably slower.
-
Catalytic Converters:
- Significantly reduce vehicle emissions, thus supporting a healthier environment.
The decomposition of hydrogen peroxide exemplifies manganese dioxide's role as a catalyst, demonstrating its capability to reduce activation energy and expedite reactions.
Lowering Activation Energy
- Catalyst: An agent that accelerates a chemical reaction while remaining unchanged.
- Catalysts remain chemically intact post-reaction, enhancing their reusability.
- They decrease activation energy by providing an alternative route for the reaction.
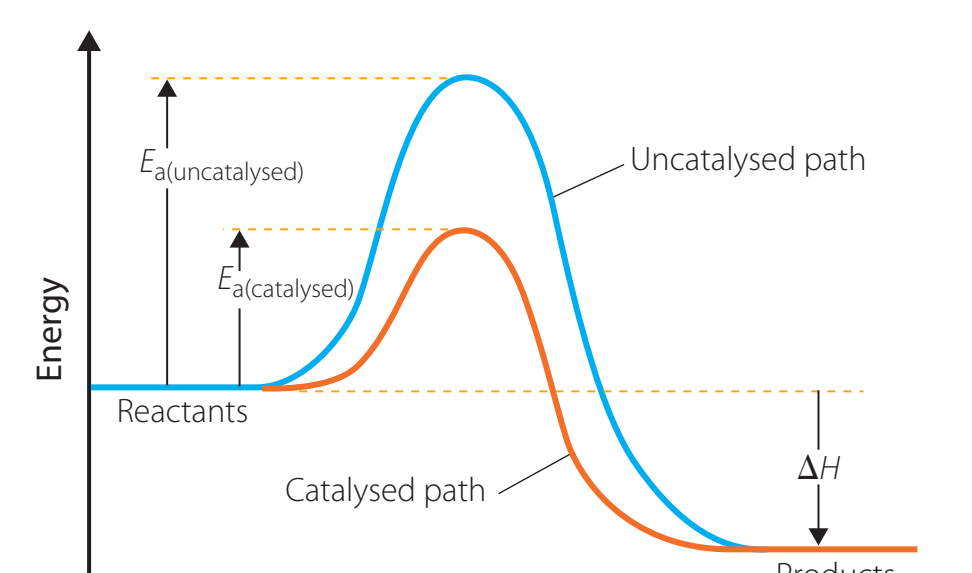
Energy Profile Diagrams
- Energy Diagrams: Visual representations that depict energy changes in both catalysed and uncatalysed reactions.
- Uncatalysed Reaction:
- Higher peak or 'hump', indicating greater activation energy.
- Catalysed Reaction:
- Lower peak, indicating reduced activation energy.
- Transition State:
- Catalysts stabilise the transition state, lowering energy demands.
- Uncatalysed Reaction:
Transition State Theory
- Theory Explanation:
- Catalysts stabilise the transition state, rendering reactions more energetically viable.
- Example: In esterification, catalysts stabilise intermediates, thereby reducing energy requirements.
Interpreting Reaction Coordinate Diagrams
Overview
- Reaction Coordinate Diagrams: Utilised in chemistry to visualise energy changes during a reaction. They aid in comprehending reaction progress, particularly when comparing catalysed and uncatalysed scenarios.
Key Term: Reaction Coordinate Diagram: A visual tool depicting energy changes throughout a chemical reaction.
Analysing Catalysed vs. Uncatalysed Reactions
- Colour-Coded Pathways:
- Use colour in diagrams to indicate catalysed (lower peaks) and uncatalysed (higher peaks) pathways.
- Pathway Comparison:
- Catalysts: Offer an easier route without modifying the final energy difference between reactants and products.
Summary Table
| Aspect | Uncatalysed Reaction | Catalysed Reaction |
|---|---|---|
| Peak Height | High (Greater Energy) | Lower (Reduced Energy) |
| Reaction Speed | Slower | Faster |
| Activation Energy | Higher | Lower |
| Final Energy Difference | Unchanged | Unchanged |
Conclusion: Catalysts reduce activation energy but do not alter the overall net energy change.
Experimental Design
Model Reaction
- Select a Model Reaction:
- Example: where manganese dioxide is utilised.
- Safety Precautions:
- Wear protective gear: goggles, gloves, and lab coats.
- Handle hydrogen peroxide cautiously to prevent skin irritation.
Data Collection Techniques
- Measurement Tools:
- Use gas syringes to accurately measure the evolved oxygen gas.
- Controlled Variables:
- Ensure consistency through water baths and maintain precise solution concentrations.
Employ digital tools for effective data logging and results.
Procedure Steps
| Step | Action | Expected Result | What to watch for |
|---|---|---|---|
| 1. Preparation | Measure and prepare reactants | Reactants prepared | Ensure precise measurement of reactants |
| 2. Execution | Start reaction by adding catalyst | Initiation of oxygen gas evolution | Observe bubble formation indicating Oxygen evolution |
| 3. Data Recording | Measure volume of O₂ at prescribed intervals | Data reflecting reaction progression | Confirm stable gas syringe readings |
Data Analysis
- Expected Results:
- Accelerated reaction rate when a catalyst is employed.
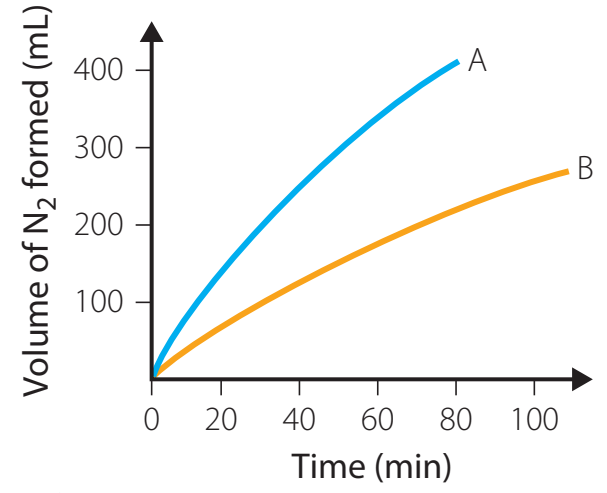
- Graphical Representation:
- Plotting volume of against time.
Statistical Analysis and Interpretation
- Organising Strategy
- Use tables and digital spreadsheets to manage data, checking for errors such as incorrect units.
Summarising Data
- Mean, Median, Mode
- Calculate these to grasp central tendencies of data sets, while standard deviation and variance highlight data dispersion.
Statistical Measures:
- Mean: Average value of a data set
- Median: Middle value in sorted data
- Mode: Most frequently occurring value
- Standard deviation: Measure of data dispersion
- Variance: Average of squared deviations from the mean
Graphical Data Analysis
- Concentration vs. Time Graphs: Illustrate catalyst impact through variations in slope.

Practice Questions with Solutions
-
Question: Calculate the mean, median, and mode from the following reaction times (in seconds): 15, 18, 20, 15, 22, 19.
Solution:
- Mean = (15 + 18 + 20 + 15 + 22 + 19) ÷ 6 = 109 ÷ 6 = 18.17 seconds
- Median: Arrange in order: 15, 15, 18, 19, 20, 22 The median is (18 + 19) ÷ 2 = 18.5 seconds
- Mode = 15 seconds (occurs twice)
-
Question: Using the graph showing oxygen evolution over time, determine how much faster the catalysed reaction reaches 50mL of oxygen compared to the uncatalysed reaction.
Solution:
- From the graph, we can see that the catalysed reaction reaches 50mL at approximately 30 seconds
- The uncatalysed reaction reaches 50mL at approximately 90 seconds
- Therefore, the catalysed reaction is 90 - 30 = 60 seconds faster, or 3 times quicker
-
Question: Explain how outliers might affect your interpretation of catalyst efficiency in an experiment.
Solution:
- Outliers could skew the mean reaction rate, making the catalyst appear more or less efficient than it actually is
- For example, if an unusually slow reaction time is recorded due to experimental error, it would decrease the mean rate and suggest the catalyst is less effective
- Best practice would be to identify outliers through statistical tests or graphical methods, then either repeat those trials or use median values which are less sensitive to outliers
500K+ Students Use These Powerful Tools to Master Chemistry - Catalysts For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
178 flashcards
Flashcards on Chemistry - Catalysts
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Chemistry - Catalysts
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes9 questions
Exam questions on Chemistry - Catalysts
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Chemistry - Catalysts
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemistry - Catalysts
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemistry - Catalysts you should explore
Discover More Revision Notes Related to Chemistry - Catalysts to Deepen Your Understanding and Improve Your Mastery
