Photo AI
Last Updated Sep 24, 2025
Temperature and Activation Energy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Temperature and Activation Energy quickly and effectively.
206+ students studying
Temperature and Activation Energy
Introduction to Collision Theory
Collision Theory: This framework explains chemical reaction rates. It is essential for predicting how reactions occur and optimising industrial processes.
- Why Do Collisions Matter?
- For a reaction to proceed, two essential conditions must be met:
- Energy Exceeding Activation Energy Barrier: Sufficient energy is necessary to surpass this threshold to initiate a reaction.
- Correct Molecular Orientation: Proper alignment of molecules is critical for reactants to successfully form products.
- For a reaction to proceed, two essential conditions must be met:
Collision Theory: Describes reactions as events where reactants must collide with adequate energy and correct orientation for success.
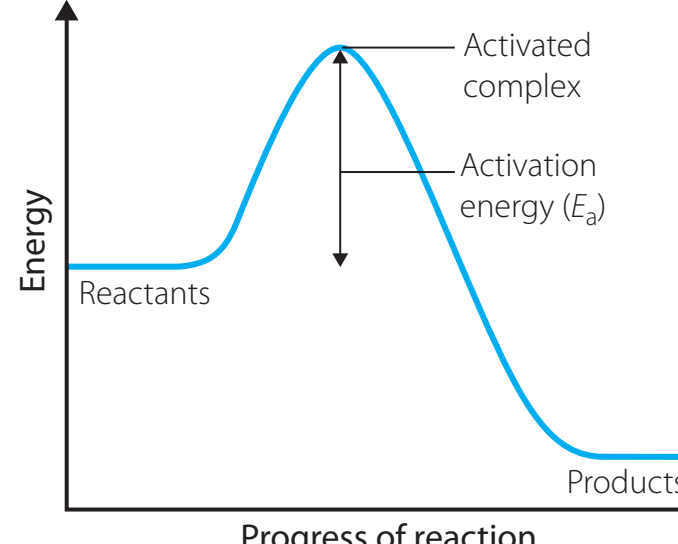
Role of Activation Energy
- Activation Energy (Ea): The minimum energy required to initiate a reaction. This energy serves as a barrier that dictates the reaction rate.
Activation Energy is a key factor in determining the feasibility of a reaction pathway. Reactants must possess sufficient kinetic energy to overcome this barrier for a successful reaction.
Key Terms
- Activation Energy: The energy necessary to initiate a reaction.
- Molecular Orientation: The spatial configuration needed for effective reactant interaction.
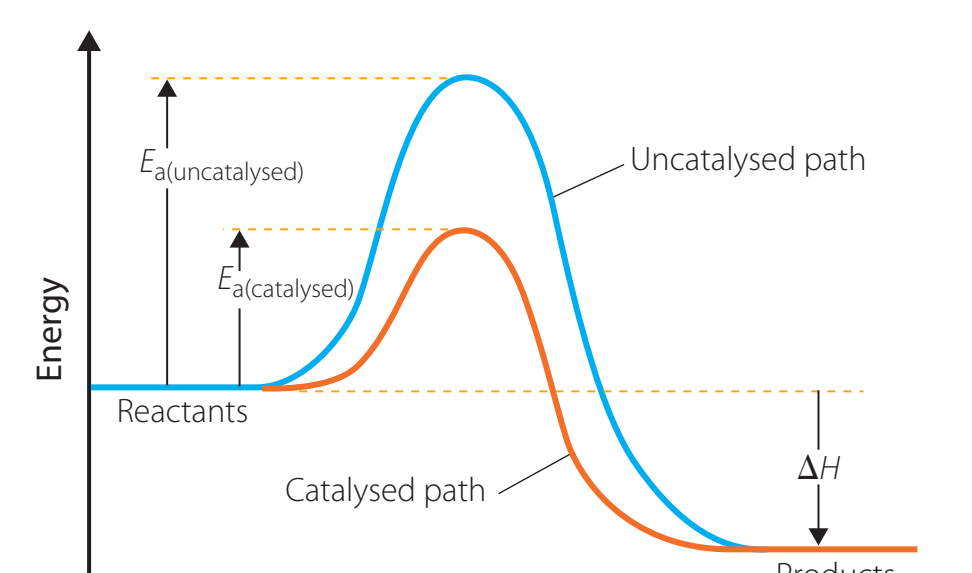
Influence of Catalysts on Reaction Rates
- Catalysts: Substances that reduce the activation energy, enabling reactions to proceed more efficiently without being consumed.
- Role of Catalysts: They offer an alternative reaction pathway, significantly enhancing the reaction rate.
Example: Enzymes act as catalysts in digestion, expediting the breakdown of food molecules.
The Effect of Temperature on Reaction Rates
- Temperature: Reflects the kinetic energy of particles. Elevated temperatures cause particles to move faster, resulting in more frequent and energetic collisions.
- Kinetic Energy and Reaction Rate: Increased kinetic energy at higher temperatures raises the collision rate, improving the likelihood of surpassing the activation energy.
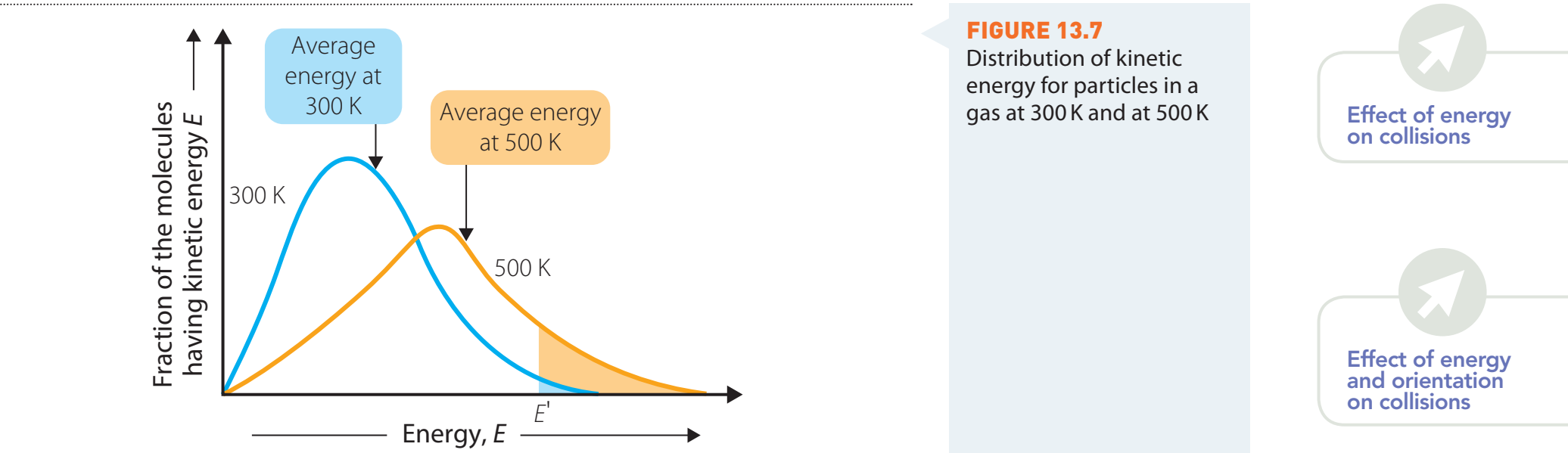
Maxwell-Boltzmann Distribution
- Illustrates how particles are distributed according to energy at varying temperatures.
- A rise in temperature generally leads to more particles possessing energy greater than the activation energy, thus speeding up the reaction.
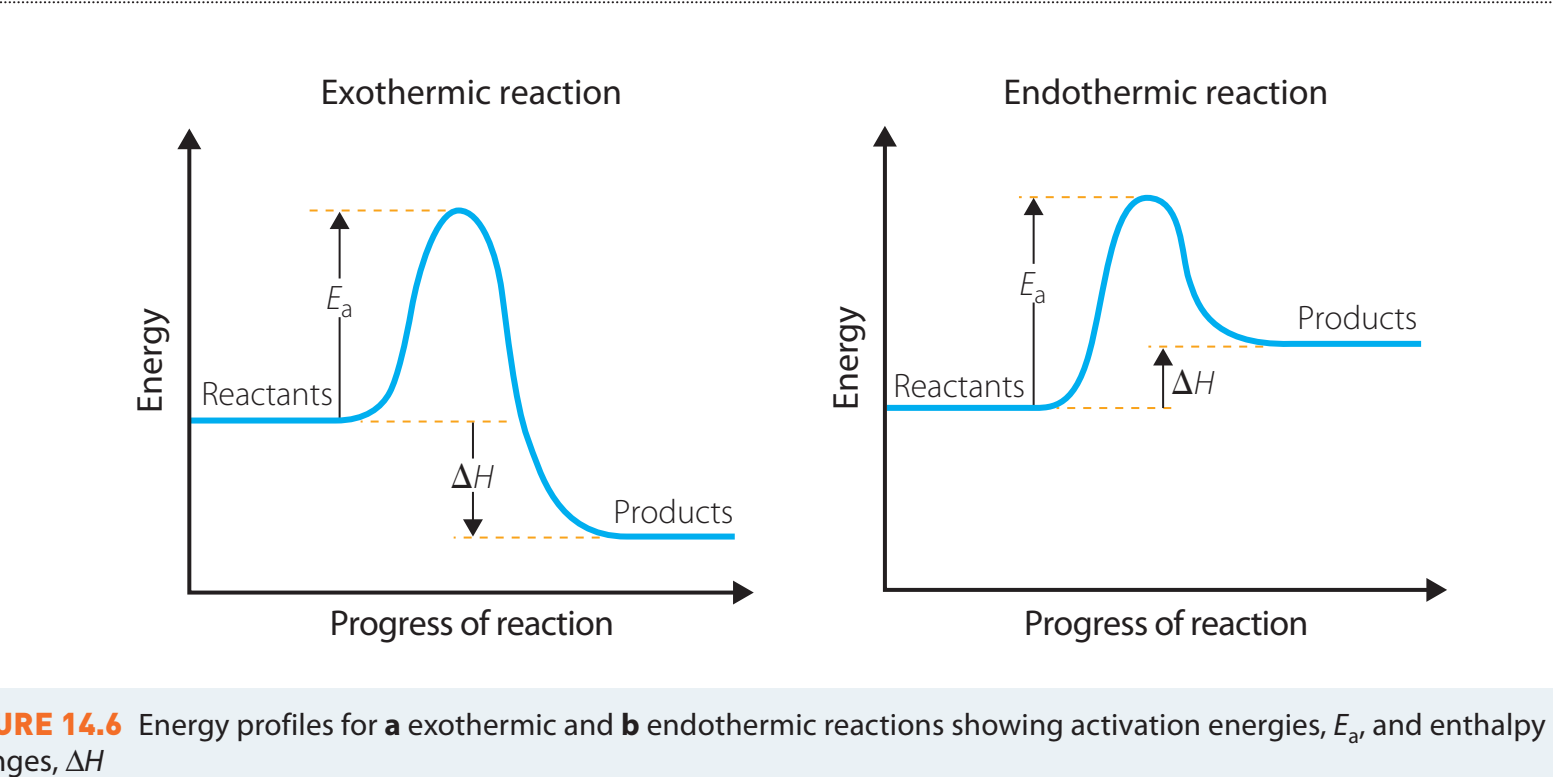
Energy Profile Diagrams
- Components:
- Activation Energy (Ea): The energy required to reach the transition state.
- Transition State: A high-energy state as reactants convert to products.
- Gibbs Free Energy Change (ΔG): The difference in energy between products and reactants, indicating whether the reaction is exothermic or endothermic.
Energy profile diagrams are instrumental in understanding the dynamics and energy transitions within reactions.
Arrhenius Equation
- Formula:
- : Reaction rate constant, which increases with temperature.
- : Frequency factor, representing the likelihood of successful collisions.
- : Activation energy.
- : Universal gas constant.
- : Temperature in Kelvin, impacting .
Molecular Orientation in Reactions
- Definition: The spatial configuration of reactant molecules that affects reaction success.
- Successful Collisions: Proper alignment of molecules is akin to fitting puzzle pieces together, vital for enabling the reaction.
Catalysts and Their Importance
- Definition and Role: Substances that accelerate reactions by offering alternative pathways. They remain unchanged after the reaction.
- Application: Extensively used in industrial and biological systems to enhance efficiency and minimise costs.
Catalysts are crucial for reducing energy barriers, facilitating faster reactions.
Activation Energy Diagrams
Conclusion
A thorough understanding of how temperature, catalysts, and molecular orientation affect chemical reactions is essential for controlling and optimising reaction rates. These principles are integral in scientific research and numerous industrial applications, significantly influencing energy efficiency and productivity.
Practice Question
- Discuss how temperature increases affect ammonia production in the Haber process.
- Consider how catalysts alter energy profile diagrams and reaction paths in your answer.
Solution: Temperature increases in the Haber process have two competing effects on ammonia production. According to the Arrhenius equation, higher temperatures increase the reaction rate by providing more molecules with energy exceeding the activation energy. However, since the reaction is exothermic (N₂ + 3H₂ ⇌ 2NH₃), Le Chatelier's principle indicates that higher temperatures favour the reverse reaction, reducing yield.
Catalysts (iron in the Haber process) create an alternative reaction pathway with lower activation energy, shown in energy profile diagrams as a lower energy barrier. This allows the reaction to proceed faster without requiring higher temperatures that would reduce yield. The catalyst doesn't change the overall energy difference between reactants and products, but significantly increases the rate at which equilibrium is reached.
500K+ Students Use These Powerful Tools to Master Temperature and Activation Energy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
178 flashcards
Flashcards on Temperature and Activation Energy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Temperature and Activation Energy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes9 questions
Exam questions on Temperature and Activation Energy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Temperature and Activation Energy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Temperature and Activation Energy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Temperature and Activation Energy you should explore
Discover More Revision Notes Related to Temperature and Activation Energy to Deepen Your Understanding and Improve Your Mastery
