Photo AI
Last Updated Sep 24, 2025
Rates of Reaction - Collision Theory Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Rates of Reaction - Collision Theory quickly and effectively.
422+ students studying
Rates of Reaction - Collision Theory
This document provides an essential overview of reaction rates and collision theory, highlighting the factors that affect chemical reactions.
Explanation of Reaction Rates
- Reaction Rate: The rate at which a chemical reaction proceeds.
- It indicates the speed at which reactants are converted to products.
- Understanding reaction rates is vital for optimising production, managing pollutants, and comprehending biochemical processes in both industrial and biochemical contexts.
Reaction rates are fundamental for regulating processes in industries and biochemistry.
Definition of Key Terms
| Term | Definition |
|---|---|
| Reaction Rate | The rate at which reactants are converted into products. |
| Reactants | The initial substances involved in a chemical reaction. |
| Products | The substances produced as a result of a chemical reaction. |
- Reaction Rate: Conversion speed of reactants to products.
- Reactants: Initial substances in a reaction.
- Products: Final substances formed from a reaction.
Factors Affecting Reaction Rates
Key Influencing Factors:
-
Concentration:
- Higher concentration increases the likelihood of collisions, thus accelerating the reaction rate.
-
Temperature:
- Elevated temperatures increase particle energy, promoting more collisions and enhancing the reaction rate.
-
Catalysts:
- Catalysts facilitate faster reactions by reducing the required activation energy without being consumed.
-
Particle Size:
- Smaller particles offer a greater surface area, which enhances the frequency of collisions.
-
An understanding of these factors is linked to collision theory.

Introduction to Collision Theory
Collision Theory: Reactions occur when particles collide with adequate energy and the correct orientation.
- Effective collisions are imperative for accelerating reactions.
Collision Theory Basics
- Collision Occurrence: Only colliding particles have the potential to react.
- Sufficient Energy: Particles must have sufficient energy to surpass the activation energy barrier.
- Proper Orientation: Accurate alignment of particles during collisions is crucial.
Effective collisions: Fulfil all criteria, resulting in a chemical reaction.
Molecular Collisions and Orientation
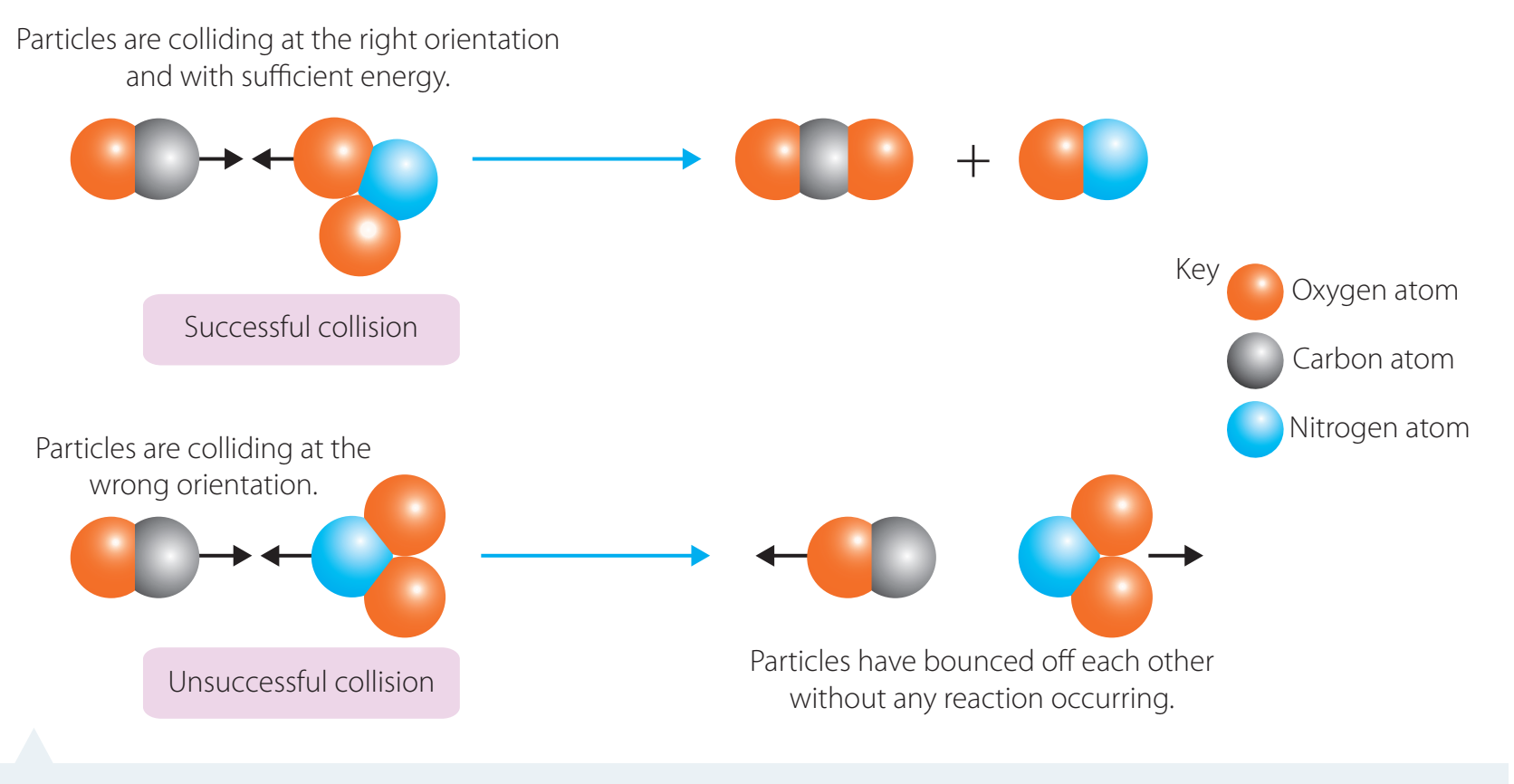
- Molecular Orientation: The arrangement of molecules plays a crucial role in determining the success of collisions, akin to the alignment of puzzle pieces.
- Correct alignment, combined with sufficient energy, is required for effective reactions.
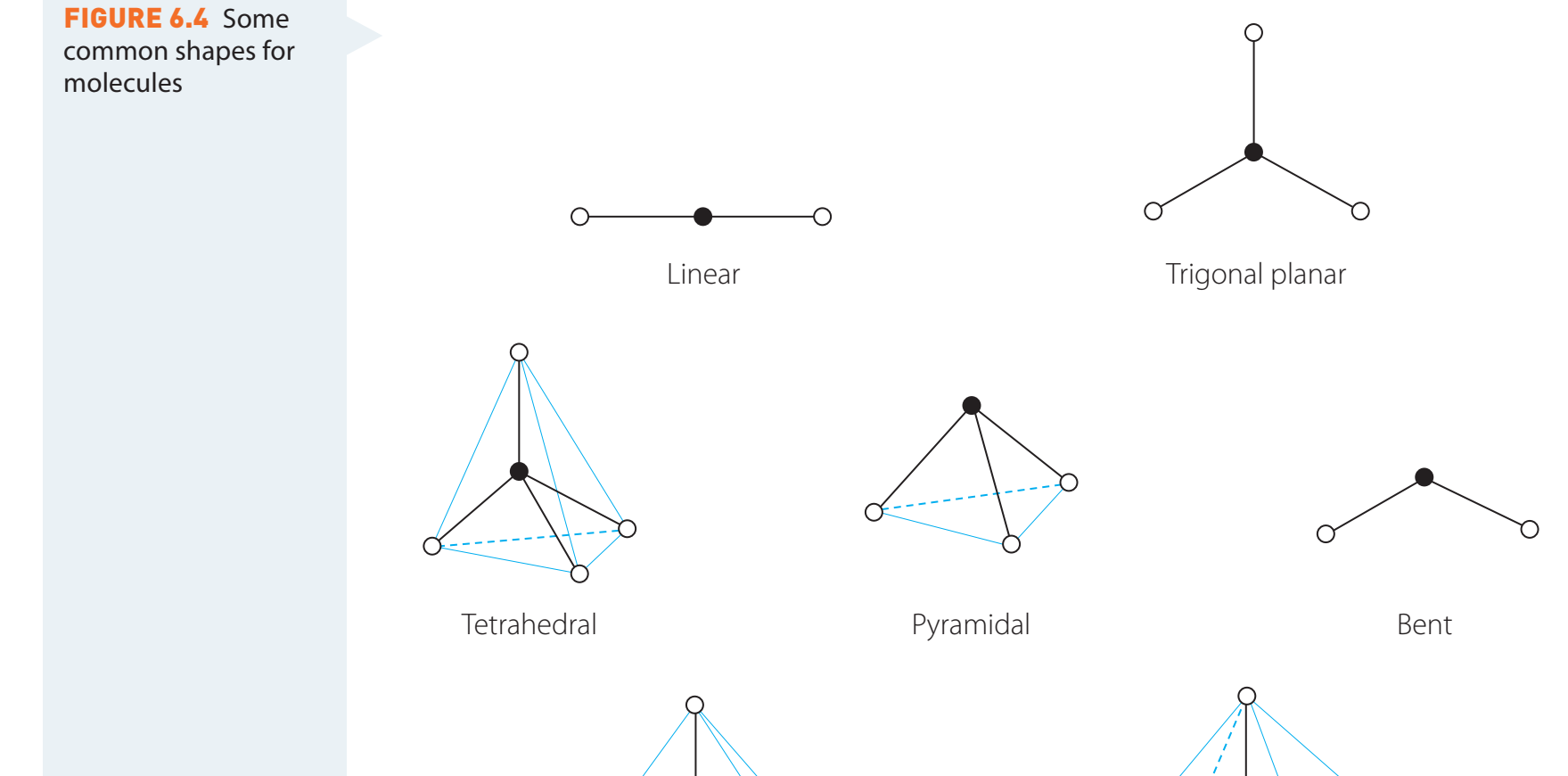
Impact of Molecular Geometry
- Molecular Geometry: The shape of molecules affects collision pathways.
- CO₂ (linear): Permits direct collisions.
- H₂O (bent): Necessitates angle navigation for effective collisions.
Visual Representation:
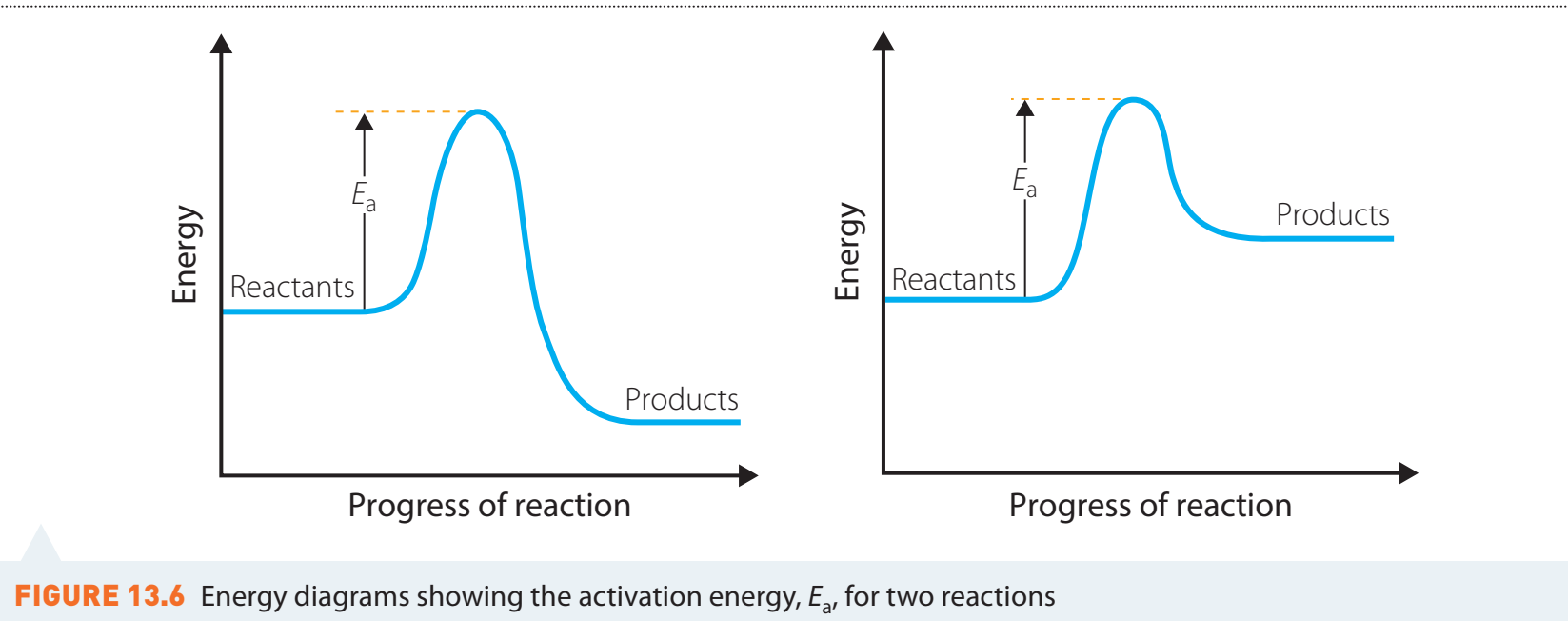
Activation Energy
Understanding Activation Energy
- Activation Energy: The minimum amount of energy required for a chemical reaction. It represents the energy barrier that must be overcome.
Activation Energy: The minimal energy necessary for reactants to initiate a reaction.
Activation Energy as an Energy Barrier
- Energy Barrier: Determines reaction rates.
- Higher activation energy results in slower reactions.
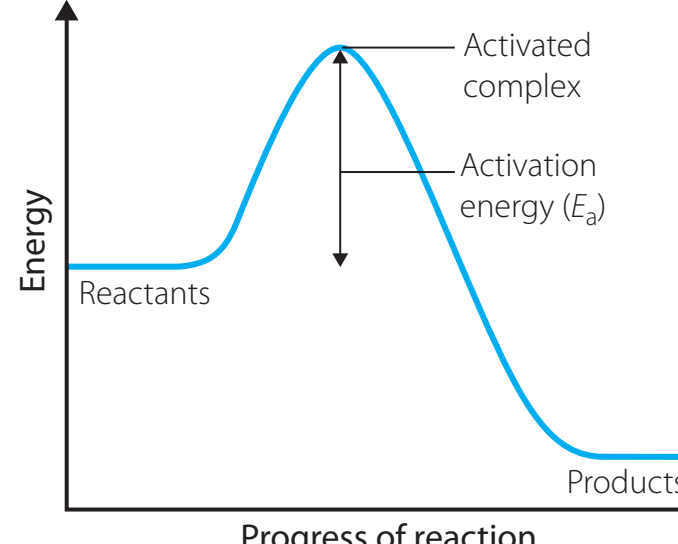
- Effects of Catalysts and Temperature:
- Catalysts decrease the activation energy.
- Temperature increases the energy available to reactants.
Key Points to Remember
-
Collision Theory Criteria: Mastery of these criteria is essential:
- Sufficient energy: Particles must possess adequate energy for reactions.
- Proper orientation: Correct alignment is crucial.
- Physical collision: A tangible collision is necessary.
-
Activation Energy:
- Functions as an energy barrier.
- Catalysts lower this barrier, accelerating reactions.
Catalysts lower activation energy, thereby increasing reaction speed—a fundamental concept for exams.
Common Pitfalls to Avoid
- Molecular Orientation Misunderstanding:
- Correct orientation is vital.
- Energy Profile Interpretation:
- Identify sections representing activation energy.
Practical Applications
- Real-world examples, such as enzyme catalysts, provide valuable insights.
- Use annotated diagrams for clarity.
- Conduct simple experiments to reinforce the effects of temperature and catalysts.
Real-world applications: Industrial processes often utilise enzyme or industrial catalysts.
Questions and Solutions
Multiple Choice Question
Question: What does the collision theory state about reactions?
Options:
- A. Reactions occur if reactants have proper orientation and energy.
- B. All reactions are spontaneous at room temperature.
- C. Temperature does not affect rates.
- D. Catalysts are required for all reactions.
Solution: A. According to collision theory, chemical reactions occur when particles collide with both the proper orientation and sufficient energy to overcome the activation energy barrier.
Calculation Problem
Question: Calculate activation energy
Given:
- Frequency factor s⁻¹
- Rate constant s⁻¹
- Temperature K
- Gas constant J mol⁻¹ K⁻¹
Formula:
Solution:
-
Substitute the values into the formula:
-
Calculate the natural logarithm:
-
Complete the calculation: J mol⁻¹
-
Convert to kJ mol⁻¹: kJ mol⁻¹
Therefore, the activation energy is 90.4 kJ mol⁻¹.
500K+ Students Use These Powerful Tools to Master Rates of Reaction - Collision Theory For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
178 flashcards
Flashcards on Rates of Reaction - Collision Theory
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Rates of Reaction - Collision Theory
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes9 questions
Exam questions on Rates of Reaction - Collision Theory
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Rates of Reaction - Collision Theory
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Rates of Reaction - Collision Theory
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Rates of Reaction - Collision Theory you should explore
Discover More Revision Notes Related to Rates of Reaction - Collision Theory to Deepen Your Understanding and Improve Your Mastery
