Photo AI
Last Updated Sep 24, 2025
Reaction Rate Factors Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Reaction Rate Factors quickly and effectively.
203+ students studying
Reaction Rate Factors
Introduction to Reaction Rates
Definition and Mathematical Expression
- Reaction Rate: The rate at which the concentration of reactants or products changes over time.
- Role: Crucial for predicting and managing chemical processes.
- Mathematical Expression:
- Rate is defined by .
- Sign Indicators: A positive sign denotes product formation, while a negative sign denotes reactant consumption.
Everyday Scenario: Simmering at low heat preserves flavours and prevents burning, demonstrating temperature's effect on reaction rates.
Significance of Studying Reaction Rates
- Importance: Facilitates the management of chemical transformations across various sectors.
- Industries: Pharmaceuticals (optimising synthesis), Food Preservation (slowing degradation).
Examples of Control:
- Pharmaceuticals: Drugs are adjusted for optimal efficacy.
- Food Preservation: Refrigeration delays spoilage processes.
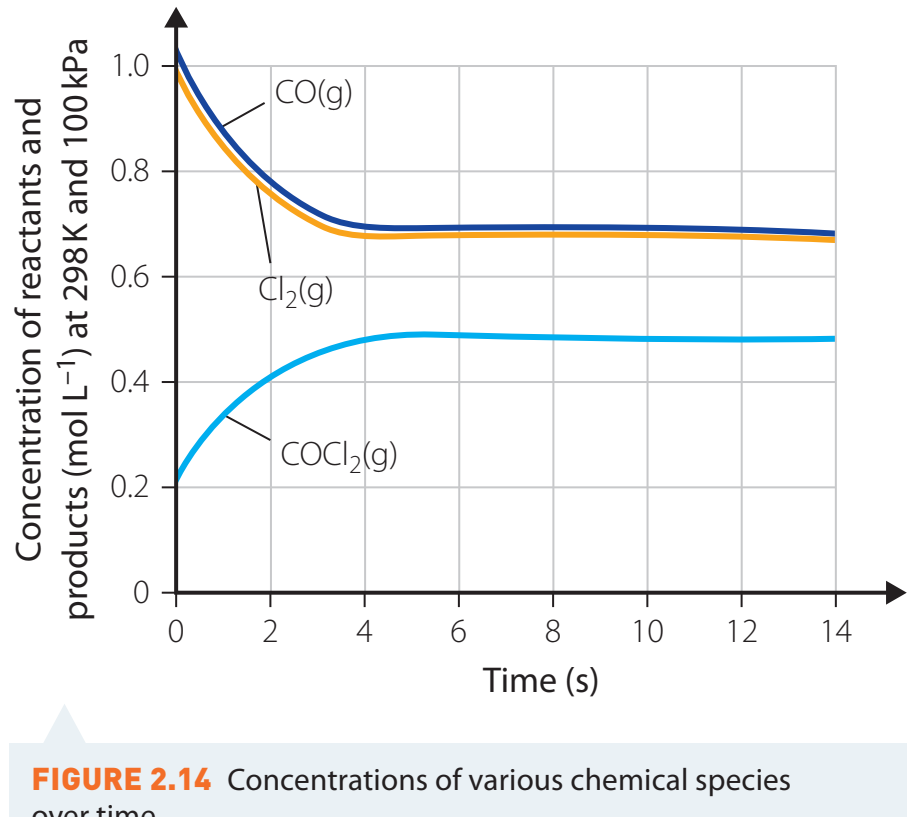
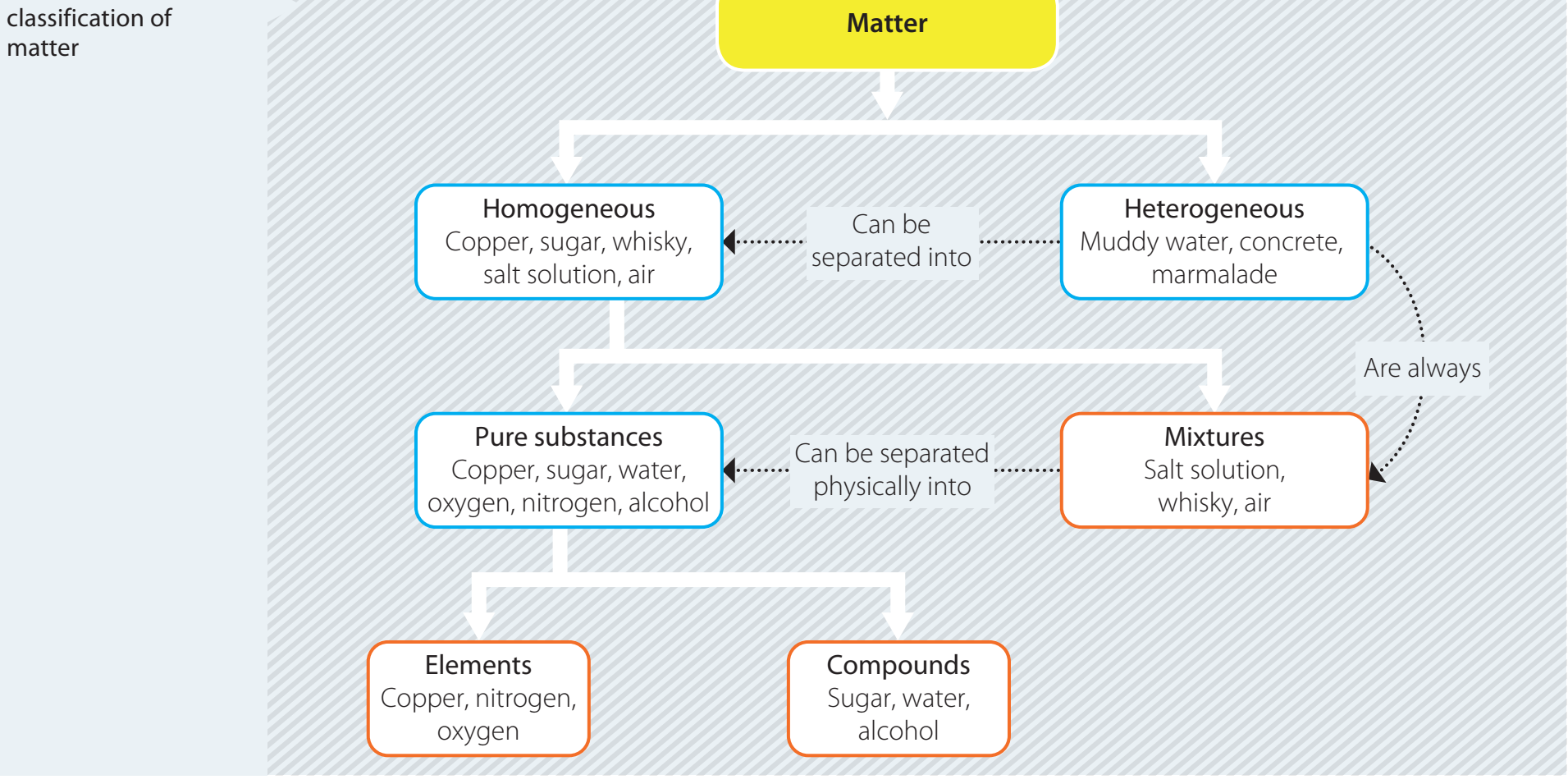
Reaction Rate Curve
- Diagram Interpretation
- The diagram depicts the concentration change over time.
- Segments:
- Initial State: Starting point of the reaction.
- Reaction Progress: Period with observable concentration changes.
- Final State: Reaction completion.
- Plateau: Rate stabilisation, indicating reaction conclusion.
Introduction to Influencing Factors
- Key Factors Affecting Rates:
- Temperature: Higher temperatures accelerate reactions; e.g., quicker cooking times.
- Surface Area: Greater area expedites reactions; e.g., chopped apples brown faster.
- Concentration: Increased concentration leads to a higher reaction rate.
- Catalysts: Speed up reactions without being consumed; e.g., yeast helps dough rise more quickly.
These concepts lay the groundwork for further detailed investigation in following sections.
Collision Theory and Temperature Impact
Collision Theory: Reactions occur when particles collide with adequate energy and suitable orientation.
-
Kinetic Energy and Temperature:
- Increased temperature boosts particle speed, comparable to cars accelerating on a motorway.
- More frequent and energetic collisions result in elevated reaction rates.
-
Effective Collisions:
- Higher temperatures augment collision frequency and effectivity.
- Imagine smoothly merging traffic as an analogy for efficient collisions.
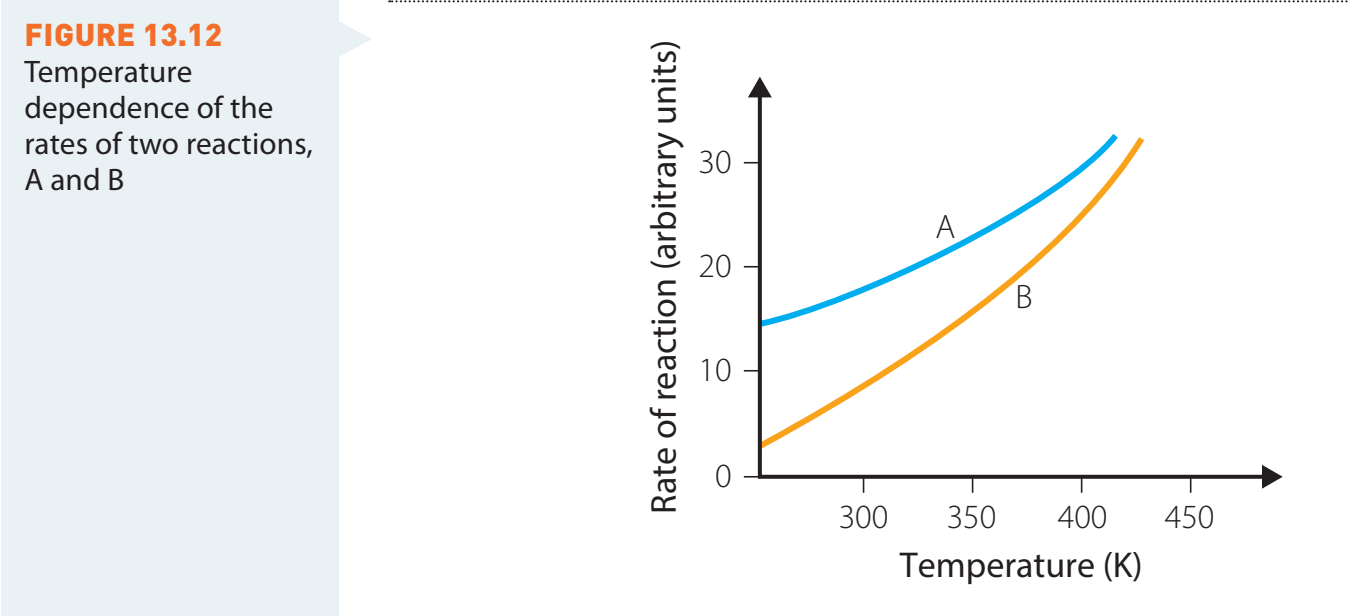
Arrhenius Equation
-
- Components:
- : Reaction rate constant.
- : Pre-exponential factor related to collision likelihood.
- : Activation energy.
- : Gas constant.
- : Temperature in Kelvin.
- Components:
Example:
- Given , , , let's find at and .
Solution: For :
For :
The reaction rate constant has approximately doubled with just a 10K increase in temperature.
The Arrhenius equation shows us that even small temperature increases can significantly accelerate reaction rates. This explains why chemical reactions proceed much faster at elevated temperatures.
Surface Area
Definition
Surface Area: The total exposed area of a solid particle's surface.
- Importance: Plays a crucial role in determining the speed of chemical reactions.
- Increases collision opportunities, leading to faster reactions.
- Example: In combustion, fine powders ignite more quickly than larger chunks due to increased surface area.
Theoretical Explanation
Collision Theory: Reactions occur upon collisions of particles with sufficient energy and optimal alignment.
- Greater surface area offers more collision sites.
- Analogy: Similar to more lanes on a motorway allowing higher traffic flow, more collision sites enhance reaction rates.
Practical Experiment
Objective: Analyse surface area's effect using calcium carbonate and hydrochloric acid.
Equipment and Materials
- Beakers
- Calcium carbonate (granules & powder)
- Hydrochloric acid
- Measuring cylinder
- Stopwatch
- Safety gear (gloves & goggles)
Method
- Measure a predetermined volume of hydrochloric acid into a beaker.
- Use identical masses of granular and powdered calcium carbonate.
- Carefully add the acid to the calcium carbonate.
- Start timing the reaction, observing until effervescence ceases.
- The powder is expected to react faster due to larger surface area.
- Note: Equal masses ensure consistent comparison.
Always wear protective gloves and goggles for safety.
Data Analysis
- Data Collection: Record precise timings and observations of the reactions.
- Reliability: Perform repeated trials for data consistency.
- Graphing: Plot reaction rates as a function of surface area for comparison.
Visual Aids:
- Particle diagrams can illustrate surface exposure differences.

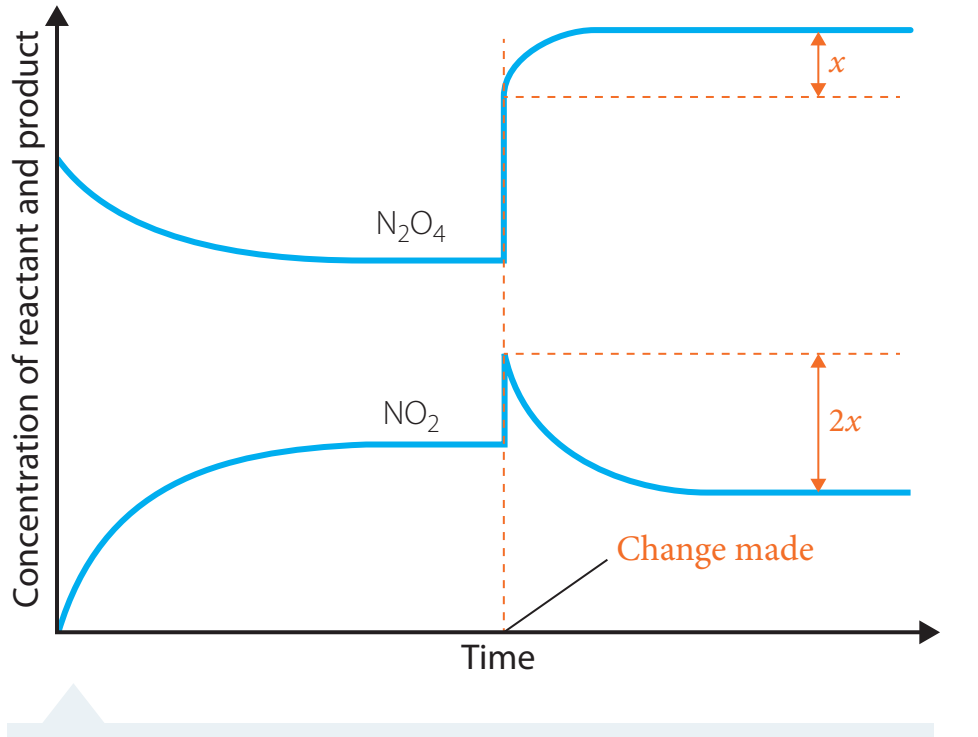
Concentration's Role in Reaction Rates
Concentration: The quantity of particles in a given volume, affecting reaction speed.
With higher concentration, increased particle presence results in accelerated reaction rates.
Theoretical Framework
Collision Theory: Higher concentrations lead to more frequent collisions.
- Particles behave like individuals in a crowded lift—higher density increases the likelihood of interactions.
- Effective collisions result in reactions.
Visualisation
-
Observe how concentration variations impact particle density and reaction likelihood.

Experimental Investigation
Objective: Examine concentration changes and their effect on reaction rates via the iodine clock reaction.
Materials: Sodium thiosulfate, potassium iodide, beakers, pipettes, and a stopwatch.
Procedure
- Prepare Reactant Solutions: Mix solutions at varying concentrations.
- Measure Reaction Times:
- Begin timing immediately after mixing starts.
- Control Variables:
- Maintain constancy in all but concentration conditions.
- Ensure precise measurements.
- Thoroughly mix solutions before timing.
Data Analysis and Presentation
-
Data Collection: Use synchronised timing for accuracy.
-
Graphical Representation:
- Plot concentration against reaction time for a clear visualisation.

Introduction to Catalysts
-
Catalyst: A substance that speeds up a chemical reaction by providing a lower activation energy pathway while remaining unchanged.
-
Importance:
- Catalysts are vital in laboratories and industries:, lowering costs and conserving energy.
- Promote sustainability through repeated use without chemical decomposition.
Catalysts ensure efficiency over time, minimising production costs and environmental impact.
Role in Reaction Mechanism
Activation Energy: The minimal energy required to start a reaction.
- Catalysts lower this threshold, enhancing reaction pace without being consumed.
Energy Profile Diagrams
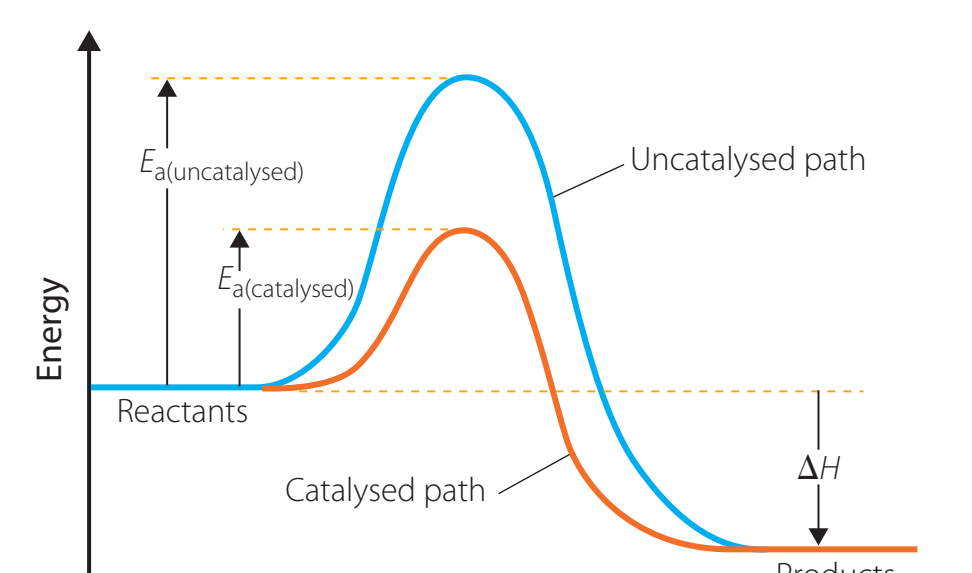
Types of Catalysts
-
Summary: Catalysts can be homogeneous or heterogeneous.
-
Homogeneous Catalysts: Share the same phase as reactants.
- Example: Enzymes in biological settings.
-
Heterogeneous Catalysts: Exist in a different phase than reactants.
- Example: Metals in the hydrogenation of gases industrially.
Experimental Demonstrations
Example Experiment:
- Reaction: Decomposition of hydrogen peroxide using manganese dioxide.
Materials & Setup
- Materials:
- Hydrogen peroxide solution
- Manganese dioxide powder
- Beaker and stirring rod
- Safety goggles and gloves
Procedure
- Put on safety goggles and gloves.
- Pour hydrogen peroxide into a beaker.
- Introduce manganese dioxide, stirring gently.
- Observe the reaction stages, such as bubble formation.
Safety Protocols:
- Avoid ingestion and skin exposure.
- Wash immediately if contact occurs.
Factors Influencing Catalyst Efficiency
Catalyst Surface Area:
- An increased surface area enhances reaction speed.
- Example: Finely powdered catalysts improve efficiency.
Temperature and Pressure:
- Raised temperatures intensify reaction rates.
- Elevated pressures positively affect gas-phase reactions.
Practical Investigations
Introduction
Practical investigations are crucial for understanding the influence of factors on reaction rates, similar to how chefs modify cooking times.
Planning the Investigation
- Define Aims and Objectives:
- Use clear bold and highlights for investigational targets.
Explore! Engage in investigations to foster scientific discovery.
Variable Identification
Independent Variable:
- Temperature
- Concentration
Dependent Variable:
- Reaction time
- Concentration change
Controlled Variables:
- Pressure
- Catalyst concentration
Setting up the Experiment
- Tools and Equipment:
- Burettes
- Pipettes
- Data Loggers
- Safety Protocols:
- Prioritise safety with goggles, gloves, and lab coats.
Conducting the Experiment
Adhere to prescribed steps to ensure accuracy and engagement during experimentation.
Data Analysis and Interpretation
- Example Analysis: Higher temperatures often lead to reduced reaction times.
- Use graphing software for interpreting collected data.
Writing the Report
- Guidance:
- Introduction: Clearly state the hypothesis.
- Methodology: Elaborate on procedures.
- Results: Incorporate visuals for data representation.
- Discussion: Discuss the significance beyond simple observations.
Reflection and Evaluation
Encourage introspection:
- "What potential biases might the design introduce?"
- "How could varying a controlled parameter provide further insights?"
Diagram Utilisation
500K+ Students Use These Powerful Tools to Master Reaction Rate Factors For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
178 flashcards
Flashcards on Reaction Rate Factors
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards17 quizzes
Quizzes on Reaction Rate Factors
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes9 questions
Exam questions on Reaction Rate Factors
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Reaction Rate Factors
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Reaction Rate Factors
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Reaction Rate Factors you should explore
Discover More Revision Notes Related to Reaction Rate Factors to Deepen Your Understanding and Improve Your Mastery
