Photo AI
Last Updated Sep 24, 2025
Metal Displacement Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Metal Displacement Reactions quickly and effectively.
448+ students studying
Metal Displacement Reactions
Introduction to Displacement Reactions
Displacement Reactions: This is a type of chemical reaction where a more reactive metal replaces a less reactive metal from its compound in solution.
-
Key Roles in understanding metal reactivity.
-
Applications include:
- Metallurgy
- Battery technology
- Corrosion science
-
Diagram:
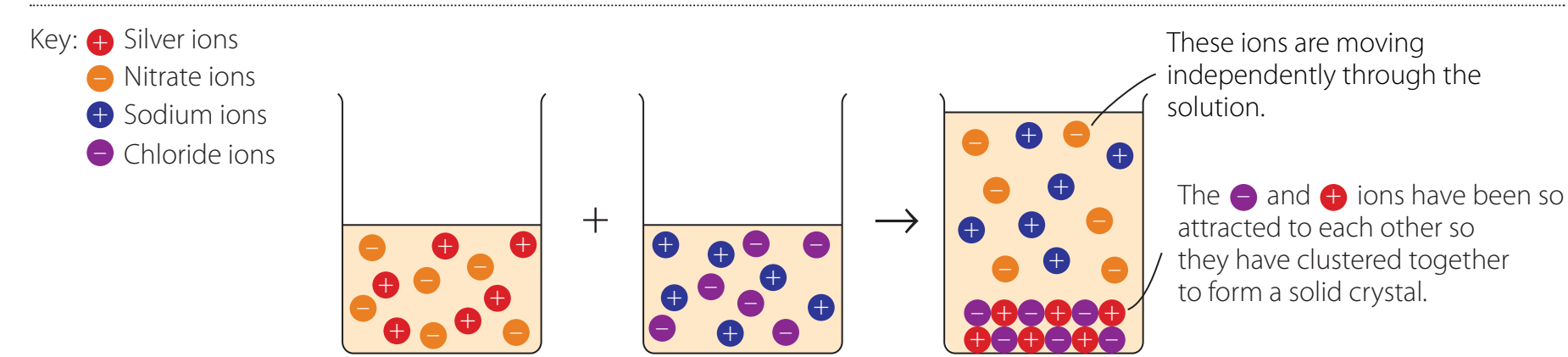
Overview of the Reactivity Series
- Reactivity Series: An ordered list of metals from most to least reactive, essential for predicting chemical behaviours and reactions.
- Significance:
- Helps predict which metals can displace others.
- Example: Potassium is the most reactive, while Gold is the least reactive.
The Reactivity Series: Important for predicting reactions and identifying potential displacements among metals.
Example Reactions and Equations
Zinc and Copper Sulphate
- Equation:
- Explanation:
- The higher reactivity of zinc allows it to displace copper from copper sulphate, demonstrating the reactivity series.
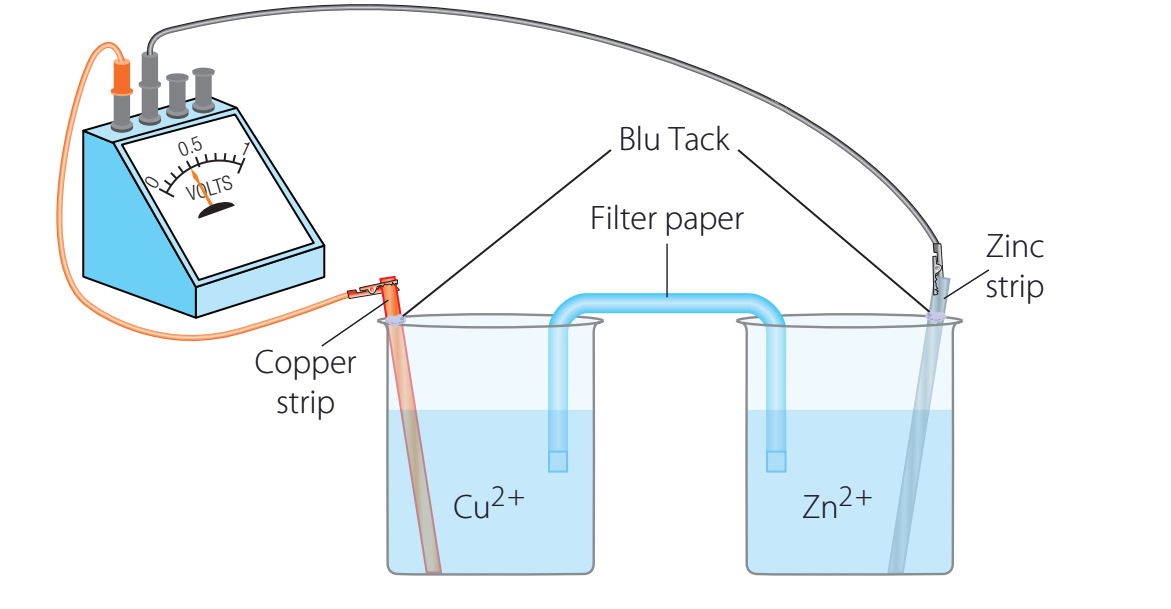
Worked Example
- Equation Breakdown:
- Reactants: Zinc (solid) and Copper Sulphate (aqueous)
- Products: Zinc Sulphate (aqueous) and Copper (solid)
- What happens: When zinc metal is placed in copper sulphate solution, the blue colour gradually fades as copper ions are replaced by zinc ions. A reddish-brown deposit of copper metal forms on the zinc surface.
Factors Affecting Metal Reactivity
Atomic Structure
- Atomic Radius: A larger radius typically means higher reactivity.
- Electron Configuration: Influences how easily electrons are lost.
- Nuclear Charge: More protons can lower reactivity by drawing electrons closer.

Ionisation Energy
- Lower ionisation energy correlates with higher reactivity.
- Example: Sodium has a lower ionisation energy than copper, making it more reactive.
Importance in Chemical and Industrial Processes
- Practical Roles:
- Fundamental in metal refining and extraction.
- Vital for metal recycling and corrosion prevention.
Why Study Displacement Reactions?
- Facilitates metal recycling.
- Aids in corrosion prevention.
- Enhances understanding of reactivity.
Historical Context and Contributors
- Notable Developments:
- Contributions by Humphry Davy and Luigi Galvani were pivotal in advancing our understanding of metal reactivity.
Practical Investigations
Experiment 1: Reactivity Series Investigation
- Equipment Needed:
- Test tubes, Bunsen burner, Tongs, Measuring cylinder
- Chemicals Used:
- Zinc, Iron, Copper, Metal salt solutions (ZnSO₄, FeSO₄, CuSO₄)
Reactivity Series: A list of metals ranked according to their ability to displace one another.
- Procedure:
- Arrange test tubes, and place each metal in its corresponding salt solution.
- Heat solutions if needed, observe changes like bubbling.
- Record observations in tables.
Safety Considerations
-
Safety Measures:
- Wear PPE: Goggles, gloves, and lab coat.
- Handle chemicals with care.
- Dispose of waste appropriately.
-
Potential Risks: Certain reactions are exothermic. Handle with caution!
Data Interpretation Guidelines
Observations
- Colour Change: A primary indicator of metal displacement.
- Precipitate Formation: Direct evidence of reactions.
- Gas Release: Rare, but can occur in reactions such as magnesium with acids.
Not all colour changes confirm displacement.
Safety and Lab Work
Prioritise safety to prevent accidents.
- PPE and Handling Protocols:
- Ensure proper use and inspection of PPE.
- Correctly label chemicals and use fume hoods.
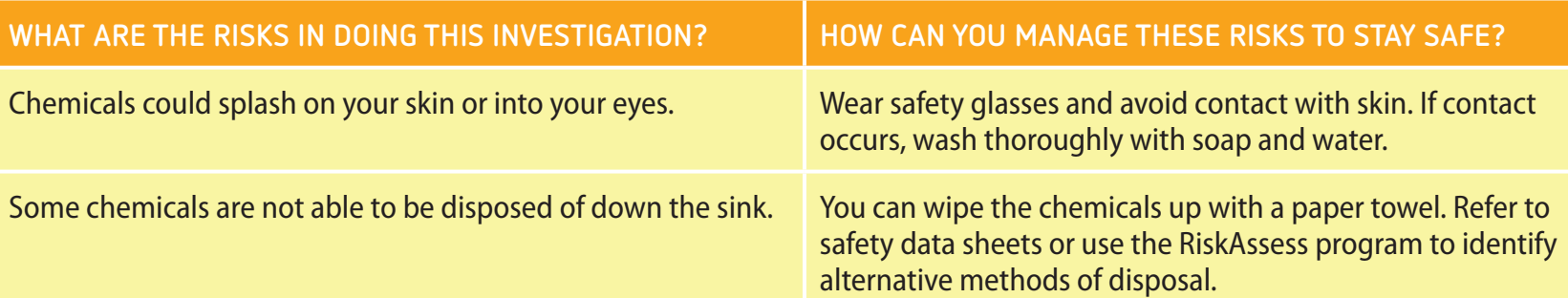
- Waste Disposal:
- Segregate and dispose of waste appropriately.
Conclusion
Understanding displacement reactions along with factors such as ion concentration, temperature, and surface area enriches the comprehension of chemical processes and industrial applications. Adhering to safety protocols and embracing analytical insights ensures effective, safe laboratory practices.
500K+ Students Use These Powerful Tools to Master Metal Displacement Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
279 flashcards
Flashcards on Metal Displacement Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Metal Displacement Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes18 questions
Exam questions on Metal Displacement Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Metal Displacement Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Metal Displacement Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Metal Displacement Reactions you should explore
Discover More Revision Notes Related to Metal Displacement Reactions to Deepen Your Understanding and Improve Your Mastery
