Photo AI
Last Updated Sep 24, 2025
Redox Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Redox Reactions quickly and effectively.
249+ students studying
Redox Reactions
This guide is specifically crafted for Year 11 students to explore the fundamental aspects of redox chemistry. It includes critical definitions, reaction examples, and essential topics such as oxidation numbers and the reactivity series. A thorough understanding of these subjects is pivotal for exams and for grasping the principles of reactive chemistry in practical applications.
Introduction
Master these now and excel in your exams later! Understanding redox chemistry is crucial for Year 11 exams. Key concepts include electron transfer processes and reactivity predictions, which lay the groundwork for robust problem-solving skills.
Key Definitions and Concepts
- Oxidation: the loss of electrons.
- Reduction: the gain of electrons.
Oxidation: the loss of electrons; Reduction: the gain of electrons
Mnemonic: OIL RIG (Oxidation Is Loss; Reduction Is Gain).

Purpose of Redox Equations
- Electron Transfer: Critical for balancing chemical equations and predicting reactions.
- Identify Electron Donors and Acceptors: Essential for understanding reaction mechanisms.
Redox Equations: These involve the electron transfer between different substances.
Understanding Electron Transfer
- Electron transfer plays a vital role in all chemical reactions. Tracking electron flow enhances the understanding and anticipation of chemical behaviour.
Grasping electron flow is indispensable for real-world reaction mechanics.
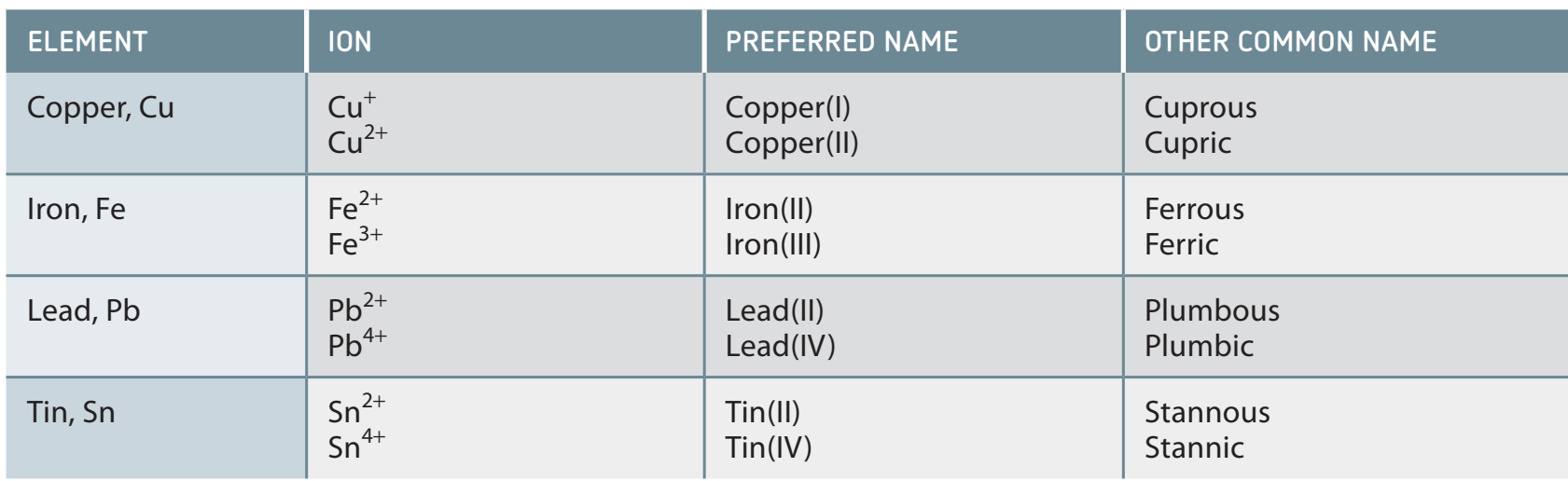
Oxidation Numbers
- Oxidation numbers assist in identifying electron transfer in redox reactions.
- Key rules:
- The oxidation number is zero in elemental form.
- In compounds, the sum of oxidation numbers is zero.
- In ions, the sum equals the charge of the ion.
Example Reactions
Magnesium and Oxygen Reaction
- Steps:
- Step 1: Electrons are transferred from magnesium to oxygen.
- Step 2: Magnesium undergoes oxidation (loss of electrons).
- Step 3: Oxygen undergoes reduction (gain of electrons).
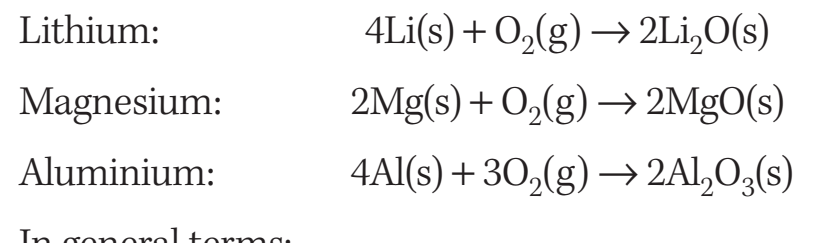
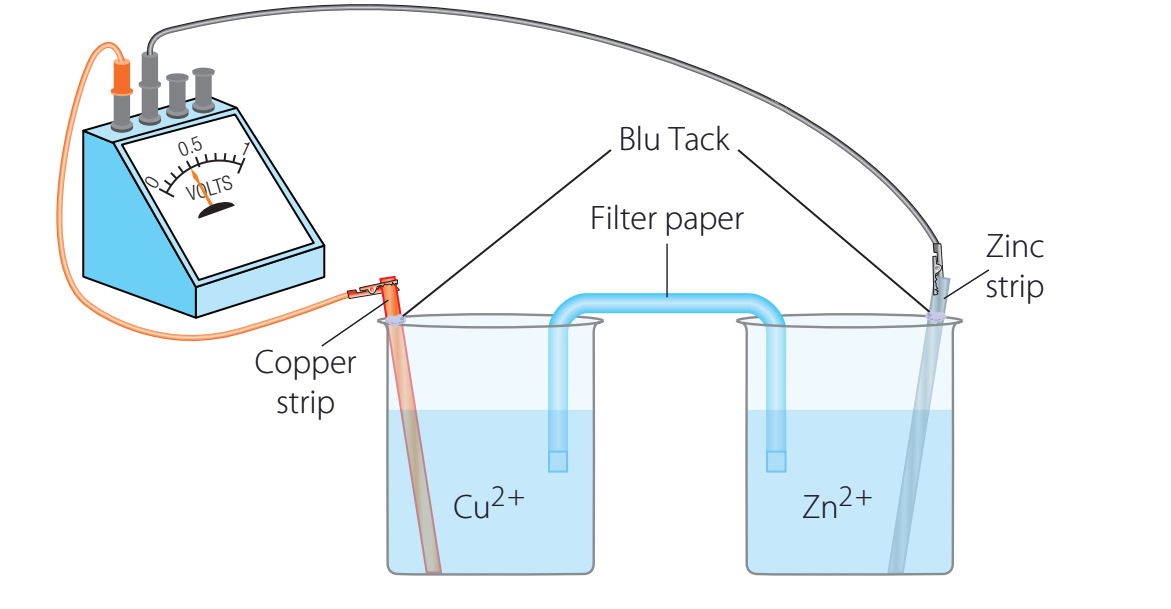
Zinc and Copper Ion Reaction
- Steps:
- Step 1: Zinc donates electrons to copper ions.
- Step 2: Zinc is oxidised; copper undergoes reduction.
Reactivity Series
Reactivity Series: This is a hierarchal list of metals ordered by reactivity, important for predicting metal reactions with substances like water and acids. It is crucial for understanding corrosion resistance and choosing suitable materials for various uses.
Criteria for Ranking Metals
- Reactions with Water: Potassium reacts very vigorously, while gold shows no reaction, indicating lower reactivity.
- Reactions with Acids: Zinc reacts with dilute hydrochloric acid, displaying its reactivity.

Constructing Half-equations
Steps
- Identify Components: Determine the oxidised and reduced species through their changes in oxidation states.
- Balance Mass and Charge: Employ H, OH, and HO for balance.
- Combine Half-equations: Ensure electron cancellation before merging.

Practice Examples
- Zinc and Hydrochloric Acid:
- Equation: Zn + 2H → Zn + H
- Solution: Zinc is oxidised (Zn → Zn + 2e) while hydrogen ions are reduced (2H + 2e → H)
- Magnesium and Oxygen:
- Equation: 2Mg + O → 2MgO
- Solution: Magnesium is oxidised (Mg → Mg + 2e) while oxygen is reduced (O + 4e → 2O)
Common Misconceptions
- Oxidation Misunderstanding: It is not solely about oxygen; rather, it involves electron transfer.
An accurate understanding of electron flow is essential.
- Clarification example: When iron rusts, the focus is on electron transfer to oxygen, not simply the addition of oxygen.
Strategy for Avoiding Mistakes
- Thoroughly review electron pathways.
- Regularly practise half-equations to ensure accurate balancing and combination.
Exam Preparation Tips
Summary
- Emphasise systematic problem-solving through diagrammatic aids and mnemonics.
Prevent common errors by practising systematically. Utilise past papers and credible online materials for practice.
- Weekly Checklist:
- Memorise mnemonics.
- Review oxidation number rules.
- Consistently solve practise redox problems.
- Timed Practices: Simulate exam conditions during practice.
Remember, mastering these fundamental concepts will not only enhance your understanding but also significantly boost your exam performance!
500K+ Students Use These Powerful Tools to Master Redox Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
279 flashcards
Flashcards on Redox Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Redox Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes18 questions
Exam questions on Redox Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Redox Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Redox Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Redox Reactions you should explore
Discover More Revision Notes Related to Redox Reactions to Deepen Your Understanding and Improve Your Mastery
