Photo AI
Last Updated Sep 24, 2025
Metal Reactivity Trends Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Metal Reactivity Trends quickly and effectively.
291+ students studying
Metal Reactivity Trends
Introduction to Metal Reactivity
What is Metal Reactivity?
Metal Reactivity: Metal reactivity indicates how readily a metal engages in chemical reactions, particularly with acids, bases, and water.
- Significance: Crucial for anticipating chemical behaviour in industrial and biological settings.
Reactivity Trends in the Periodic Table
- Periodic Table Layout:
- Periods signify energy levels.
- Groups consist of elements with similar attributes.
- Trends Across the Table:
- Group Trends: Reactivity increases as we progress down groups due to growing atomic size and added electron shielding.
- Period Trends: Reactivity decreases across periods as nuclear charge augments without additional shielding.
Key Factors Influencing Metal Reactivity
Ionisation Energy
Ionisation Energy: The energy necessary to remove an electron from an atom.
- Lower ionisation energy implies that electrons are more easily detached, resulting in heightened reactivity.
Atomic Radius
Atomic Radius: The span from the nucleus to the most distant electrons.
- A larger radius often leads to increased reactivity because outer electrons are less securely bound.
Electronegativity
Electronegativity: The capability of an atom to attract electrons.
- Metals with reduced electronegativity exhibit greater reactivity.
Exceptions and Anomalies
- Transition Metals: Do not strictly conform to trends due to intricate electron configurations.
- Example: Copper has a distinctive electron configuration resulting in low reactivity.
- Copper's filled d-subshells contribute to its stability.
Diagram and Visual Aid
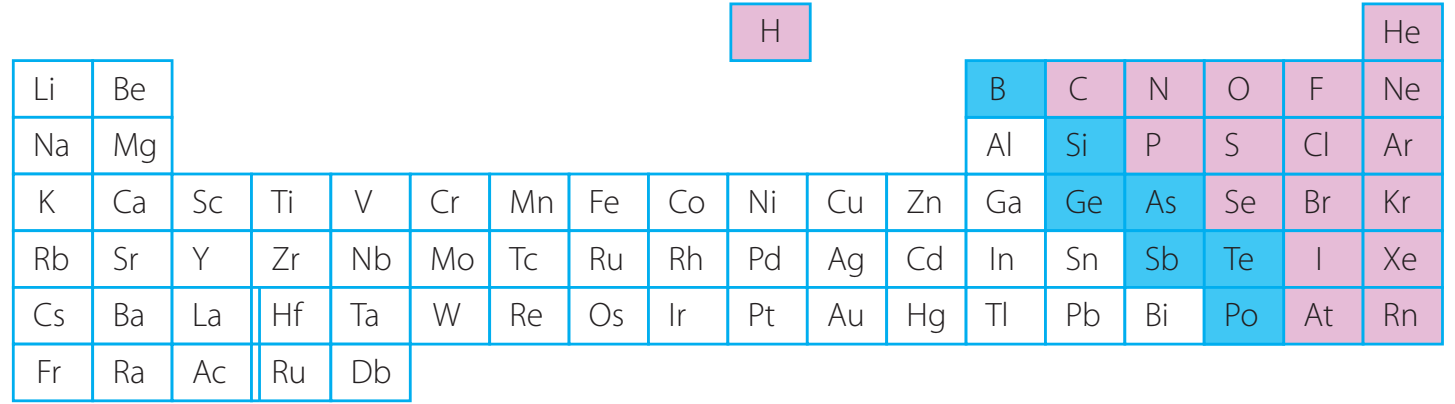 Utilise the diagram to pinpoint reactivity trends; colouration indicates differing levels of reactivity.
Utilise the diagram to pinpoint reactivity trends; colouration indicates differing levels of reactivity.
Ionisation Energy and Metal Reactivity
Definition and Core Concept
-
Ionisation Energy: The energy needed to eject one mole of electrons from one mole of gaseous atoms.
- Fundamentalfor comprehending atomic interactions and reactivity.
-
Atomic Radius: The average distance from the nucleus to the outer electron shell.
-
Electron Shielding: Inner electron shells decrease the effective nuclear charge that outer electrons experience.
Significance in Reactivity
- How Ionisation Energy Affects Reactivity:
- Lower ionisation energy equates to easier electron detachment, thus higher reactivity.
- Example: Sodium's vigorous reaction with water is due to its low ionisation energy.
Detailed Explanation of Trends
Trends Across Periods
- Increase in Ionisation Energy:
- Arises as atomic radius diminishes and nuclear charge grows.
- Electrons are drawn closer, demanding more energyfor their removal.
Trends Down Groups
- Decrease in Ionisation Energy:
- Caused by an enlarged atomic radius and enhanced electron shielding.
- Outer electrons perceive a reduced effective nuclear charge, facilitating their removal.
Correlation with Reactivity
-
Core Connection:
- Alkali metals possess low ionisation energy, hence exhibit high reactivity.
- Reactivity Series: Used for forecasting metal interactions and displacements.
-
Worked Example:
-
Comparing ionisation energies for potassium, lithium, and caesium:
-
Example Calculation:
Ionisation energies: - $E_{\text{ion}}(\text{K}) = 418 \text{ kJ/mol}$ - $E_{\text{ion}}(\text{Li}) = 520 \text{ kJ/mol}$ - $E_{\text{ion}}(\text{Cs}) = 376 \text{ kJ/mol}$ Trend: Lower energy = Higher reactivity $$E_{\text{ion}}(\text{Cs}) < E_{\text{ion}}(\text{K}) < E_{\text{ion}}(\text{Li})$$ -
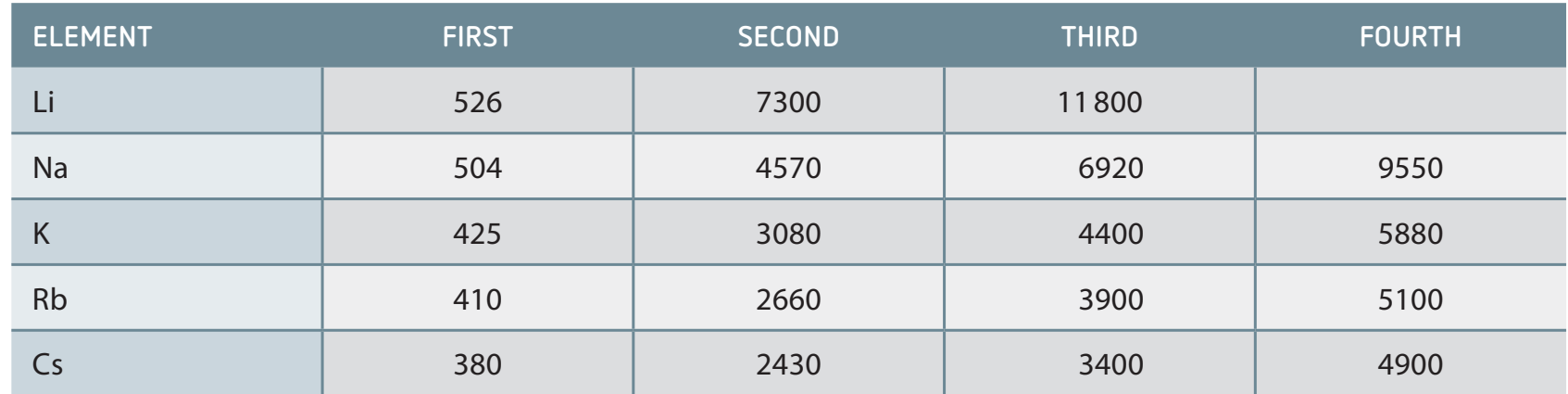
Exceptions and Anomalies
- Unique Examples:
- Magnesium (Mg) and Zinc (Zn):
- Exhibit unique trends due to filled electron subshells and electron screening effects.
- Magnesium (Mg) and Zinc (Zn):
Atomic Radius and Metal Reactivity
Atomic Radius : The space between the nucleus core and the boundary of the electron cloud.
Larger atomic radii generally equate with increased reactivity in metals.
Relevance of Atomic Radius in Metal Reactivity
- Metals with greater atomic radii tend to have higher reactivity.
- Outer electrons are held less tightly, facilitating reactions.
Explanation of Trends
-
Atomic Radius Trends:
Motion on Periodic Table Atomic Radius Trend Down a Group Increases Across a Period Decreases -
Summary:
- Moving down a group, the atomic radius augments.
- Moving across a period, the atomic radius diminishes.
- Keep these trends in mind when predicting the reactivity of elements.
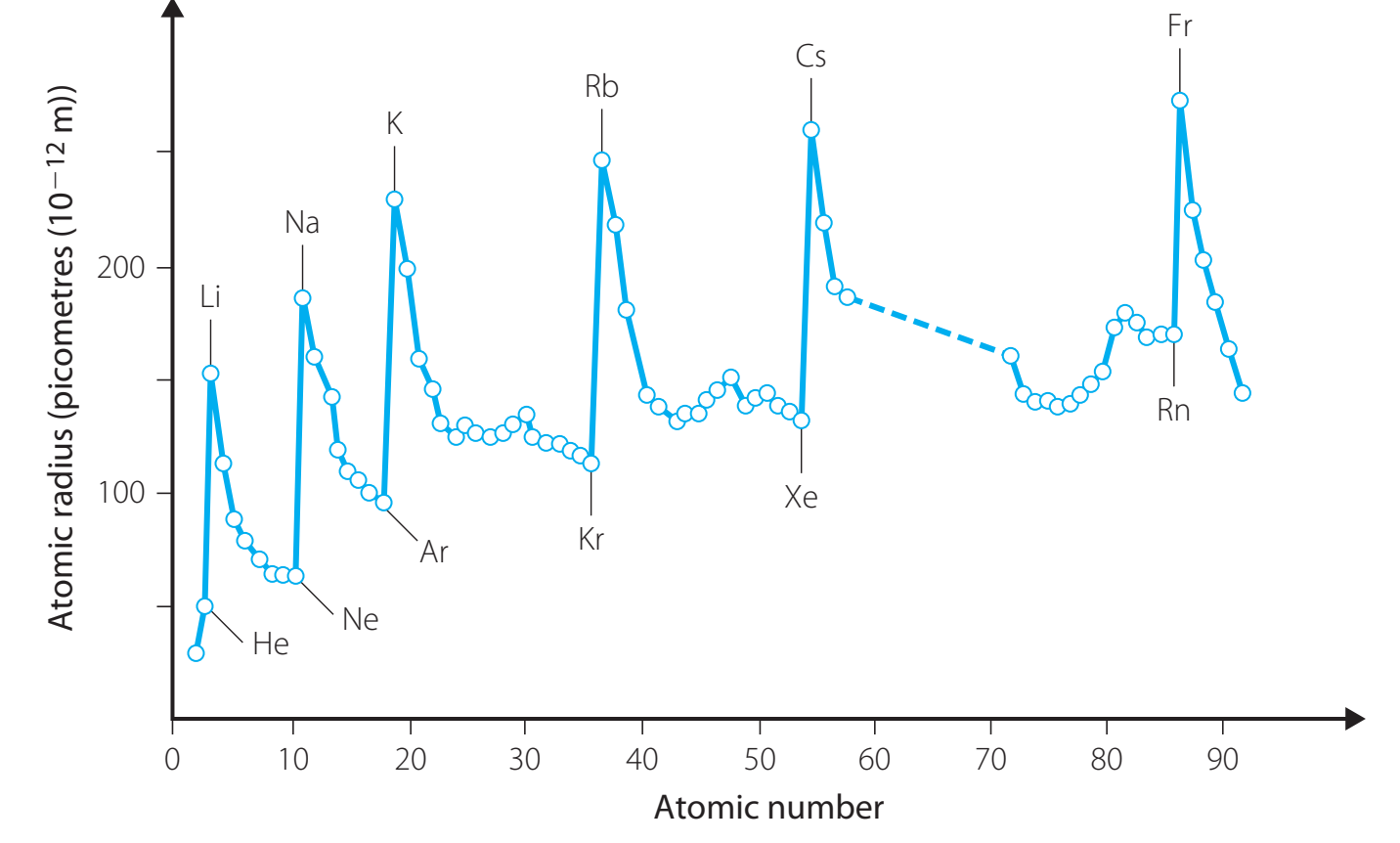 The diagram visualises the variation in atomic radius across periods and groups—consult it for a clearer representation of trends.
The diagram visualises the variation in atomic radius across periods and groups—consult it for a clearer representation of trends.
Correlation with Metal Reactivity
- Down a Group: Greater atomic radii correlate with increased reactivity.
- Example:
- Sodium vs. Caesium:
- Highlighting their different properties.
- Sodium: Possesses a smaller atomic radius; moderately reactive.
- Caesium: Features a larger atomic radius; very reactive.
- Sodium vs. Caesium:
Exceptions in Trends
- Some elements deviate from the standard due to unique features:
- Magnesium:
- Despite a smaller atomic radius, displays moderate reactivity.
- Influencing factors like ionisation energy and oxidation states play a role in these trends.
- Ionisation Energy: The force necessary to remove an electron from an atom.
- Oxidation States: The hypothetical charge an atom would have if all bonds were ionic.
Electronegativity and its Role in Reactivity
Electronegativity: Indicator of an atom's capacity to attract and hold onto electrons.
Comprehending electronegativity is essential for predicting the chemical behaviour of metals. Contrast with Ionisation Energy:
- Ionisation Energy: Energy to remove an electron from an atom.
- Electronegativity: Potential to draw electrons towards itself in a chemical bond.
Trends in Electronegativity Across the Periodic Table
-
Electronegativity Decreases Down a Group:
- Atoms acquire additional electron shells, expanding the atomic radius.
- This curtails the effectual attraction exerted on electrons.
-
Electronegativity Increases Across a Period:
- An increase in nuclear charge without an increase in shielding.
- Atoms become more adept at attracting electrons.
Observing the trend diagrams aids in grasping how these changes relate to metal reactivity.
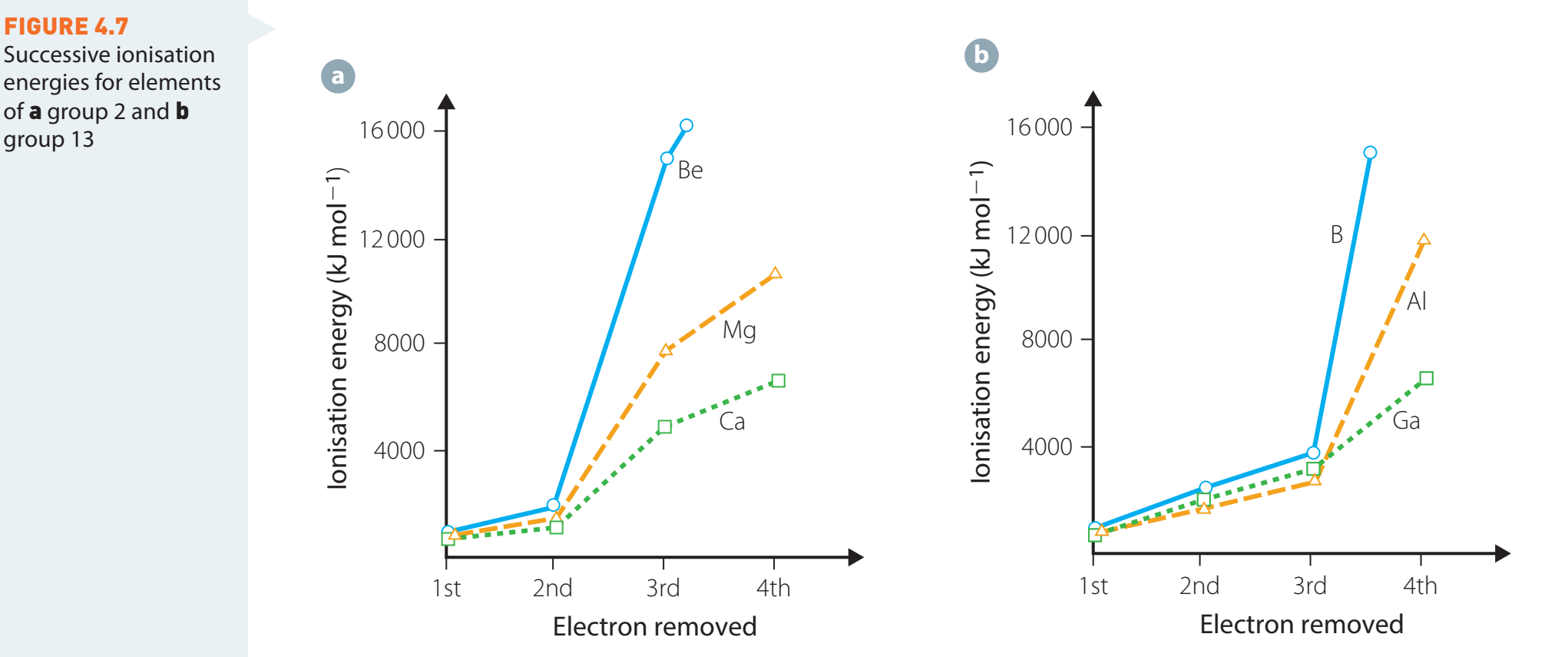
Inverse Relationship with Metal Reactivity
- Lower electronegativity is often associated with higher metal reactivity.
- Example:
- Lithium reacts intensely due to its low electronegativity.
Exceptions and Anomalies
- Transition Metals: Exhibit distinct trends owing to multiple oxidation states.
- Copper (Cu): Exhibits low reactivity compared to expected electronegativity due to filled d-orbitals.
Overview
Assessing metal reactivity involves examining multiple chemical properties. Integrating ionisation energy, atomic radius, and electronegativity offers a comprehensive understanding of metallic behaviour in reactions.
Key Insight: Synthesising trend data enhances the understanding of metal reactivity.
Activity Series of Metals
The Activity Series organises metals by their potential to displace other metals in reactions:
- Metals at the top demonstrate higher reactivity compared to those below.
- Beneficial in foreseeing reactions, particularly displacement reactions.

500K+ Students Use These Powerful Tools to Master Metal Reactivity Trends For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
279 flashcards
Flashcards on Metal Reactivity Trends
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Metal Reactivity Trends
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes18 questions
Exam questions on Metal Reactivity Trends
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Metal Reactivity Trends
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Metal Reactivity Trends
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Metal Reactivity Trends you should explore
Discover More Revision Notes Related to Metal Reactivity Trends to Deepen Your Understanding and Improve Your Mastery
