Photo AI
Last Updated Sep 24, 2025
Oxidation Numbers and Redox Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Oxidation Numbers and Redox quickly and effectively.
458+ students studying
Oxidation Numbers and Redox
Importance: Understanding oxidation numbers and redox reactions is indispensable. These concepts are vital in both academic settings and practical applications, such as industrial processes and everyday chemical interactions. Engaging with practice problems is essential for creating a solid foundation, making chemistry less abstract and more applicable.
Oxidation Numbers: Represent the hypothetical charges an atom would possess if all bonds in a compound were ionic. They are crucial for analysing chemical reactions, particularly in redox processes.
- Oxidation Numbers: Values that indicate electron transfers in chemical reactions, especially in redox processes.
- Primary Utility: Oxidation numbers assist in tracking electron transfer during reactions.
Definition and Role of Oxidation Numbers
- Oxidation Numbers: Values indicating electron transfers in chemical reactions, particularly in redox reactions.
- Use in Reactions: Essential for recognising the gain and loss of electrons, which is crucial for understanding oxidation (loss of electrons) and reduction (gain of electrons).
- Predictive Power: They aid in predicting reaction outcomes, providing insights into chemical behaviours.
Detailed Rules with Examples
-
Elements in Their Natural State: Assigned an oxidation number of 0.
- Examples: O₂, Cu.
-
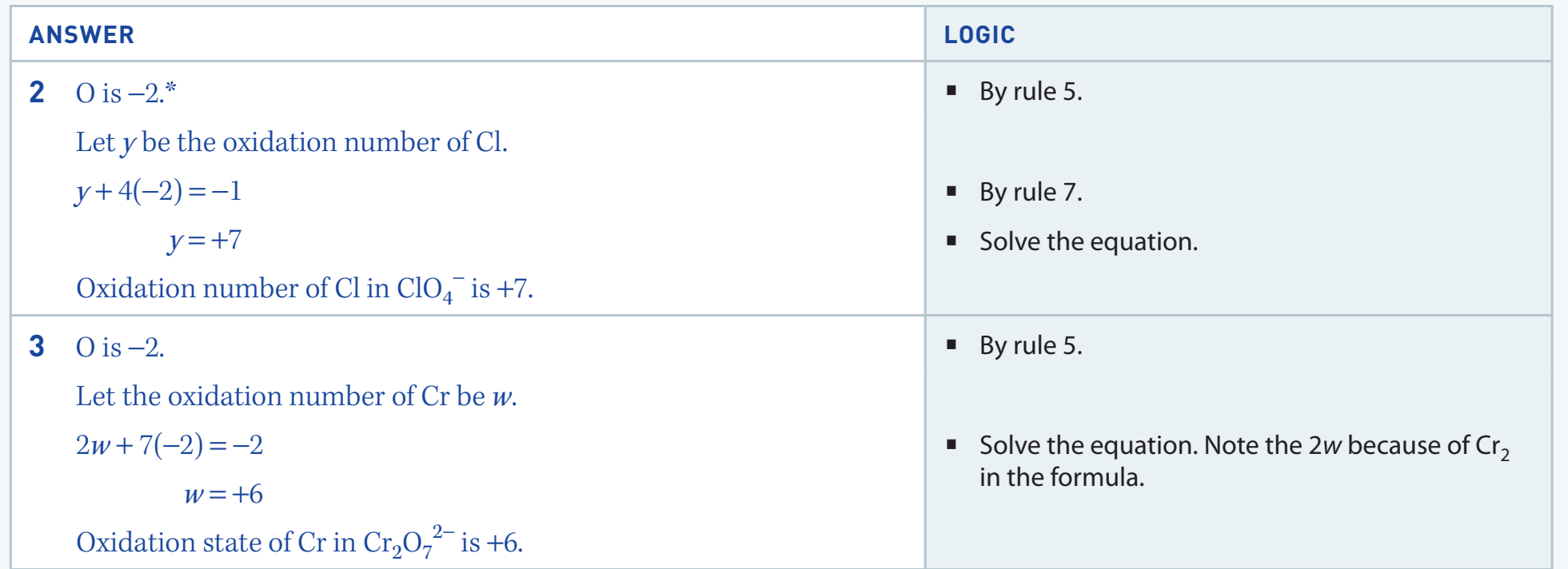
Monatomic Ions: Oxidation numbers correspond to their ionic charge.
- Examples: Na⁺ (+1), Cl⁻ (-1).
-
Fluorine: Always -1 in compounds due to its high electronegativity.
-
Hydrogen: Generally +1, except -1 in metal hydrides.
- Example: In NaH, hydrogen is -1.
-
Oxygen: Typically -2, with exceptions like -1 in peroxides such as H₂O₂.

Electron Transfer in Redox Reactions
- Redox Overview: Redox reactions involve electron transfer between chemical species.
- Correlation with Oxidation Numbers: Variations in oxidation numbers reflect electron movement, indicating which substances are oxidised (losing electrons) and which are reduced (gaining electrons).
Balancing Redox Reactions
Balancing redox reactions is crucial for students in chemistry, especially in exams and practical applications.
- Conservation Principles: Balancing ensures the conservation of mass and charge, which are fundamental in reactions.
Step-by-Step Procedure
-
Step 1: Assign Oxidation Numbers
- Example: In Zn + HCl, zinc shifts from 0 to +2, and hydrogen shifts from +1 to 0.
-
Step 2: Identify Changes in Oxidation Numbers
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
-
Step 3: Balance Electrons Transferred
- Use half-reactions to balance electron transfers effectively.
-
Step 4: Balance Everything Else
- Ensure all atoms are balanced by mass and charge.
Practising with half-reaction examples enhances understanding.
Worked Example: Balancing Redox Reactions
Problem: Balance the conversion of MnO₄⁻ to Mn²⁺ in acidic solution.
Solution:
-
Write the unbalanced equation: MnO₄⁻ → Mn²⁺
-
Assign oxidation numbers: In MnO₄⁻: Mn is +7, O is -2 In Mn²⁺: Mn is +2
-
Calculate the electron change: Mn goes from +7 to +2, gaining 5 electrons (reduction)
-
Balance with H⁺ and H₂O in acidic solution: MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
-
The balanced half-reaction shows manganese is being reduced by gaining 5 electrons.

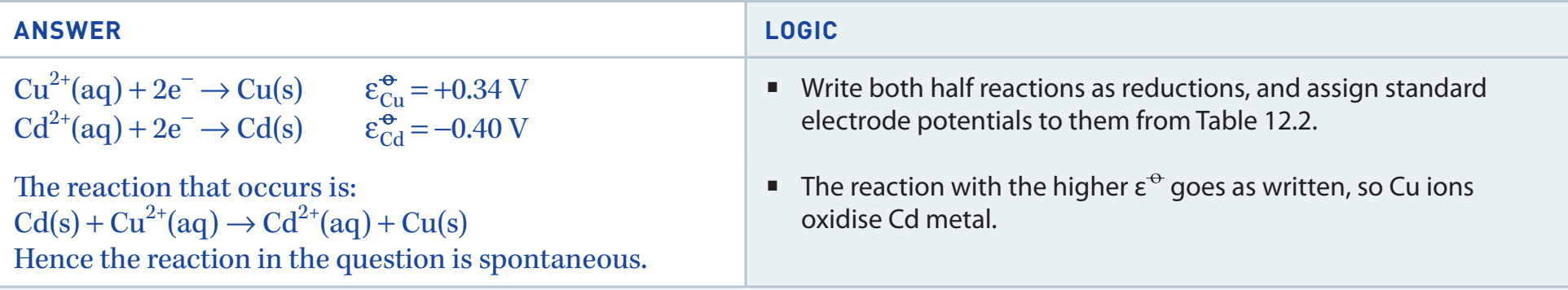
Predicting Metal Reactivity Using the Activity Series
Introduction to the Activity Series
- Activity Series: A ranking of metals based on reactivity. It predicts the outcomes of redox reactions and metal displacement events.
Activity Series: Helps determine metal reactivity and displacement potential.
- Correlation of Position and Oxidation Potential:
- Metals higher in the series lose electrons more readily.
Practical Examples
-
Example 1: Displacement Reaction
- Reaction:
- Outcome: Zinc displaces copper, being more reactive.
-
Example 2: Non-displacement Scenario
- Reaction:
- Outcome: Copper does not displace silver.

Practical Application and Examples
-
Real-world Use: Oxidation numbers are essential in areas such as metallurgy for evaluating efficiency, and environmental monitoring for tracking pollutants.
-
Example Calculations:
- In CO₂, determine carbon's oxidation number:
- Assign O = -2. With two oxygen atoms:
- Find the oxidation number of sulfur in SO₃:
- Assign O = -2, solve:
- In CO₂, determine carbon's oxidation number:
Exam Tips
- Understand and Memorise Key Rules: Familiarise yourself with rules about natural states, monatomic ions, fluorine's electronegativity, and exceptions for hydrogen and oxygen.
- Engage in Multiple Examples: Practise assigning oxidation numbers and identifying redox pairs through sample problems.
- Visualise: Utilise diagrams and visual aids to effectively understand oxidation numbers and electron transfers.
Mastering these numbers forms a foundational basis for systematic learning in chemistry.
500K+ Students Use These Powerful Tools to Master Oxidation Numbers and Redox For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
279 flashcards
Flashcards on Oxidation Numbers and Redox
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Oxidation Numbers and Redox
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes18 questions
Exam questions on Oxidation Numbers and Redox
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Oxidation Numbers and Redox
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Oxidation Numbers and Redox
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Oxidation Numbers and Redox you should explore
Discover More Revision Notes Related to Oxidation Numbers and Redox to Deepen Your Understanding and Improve Your Mastery
