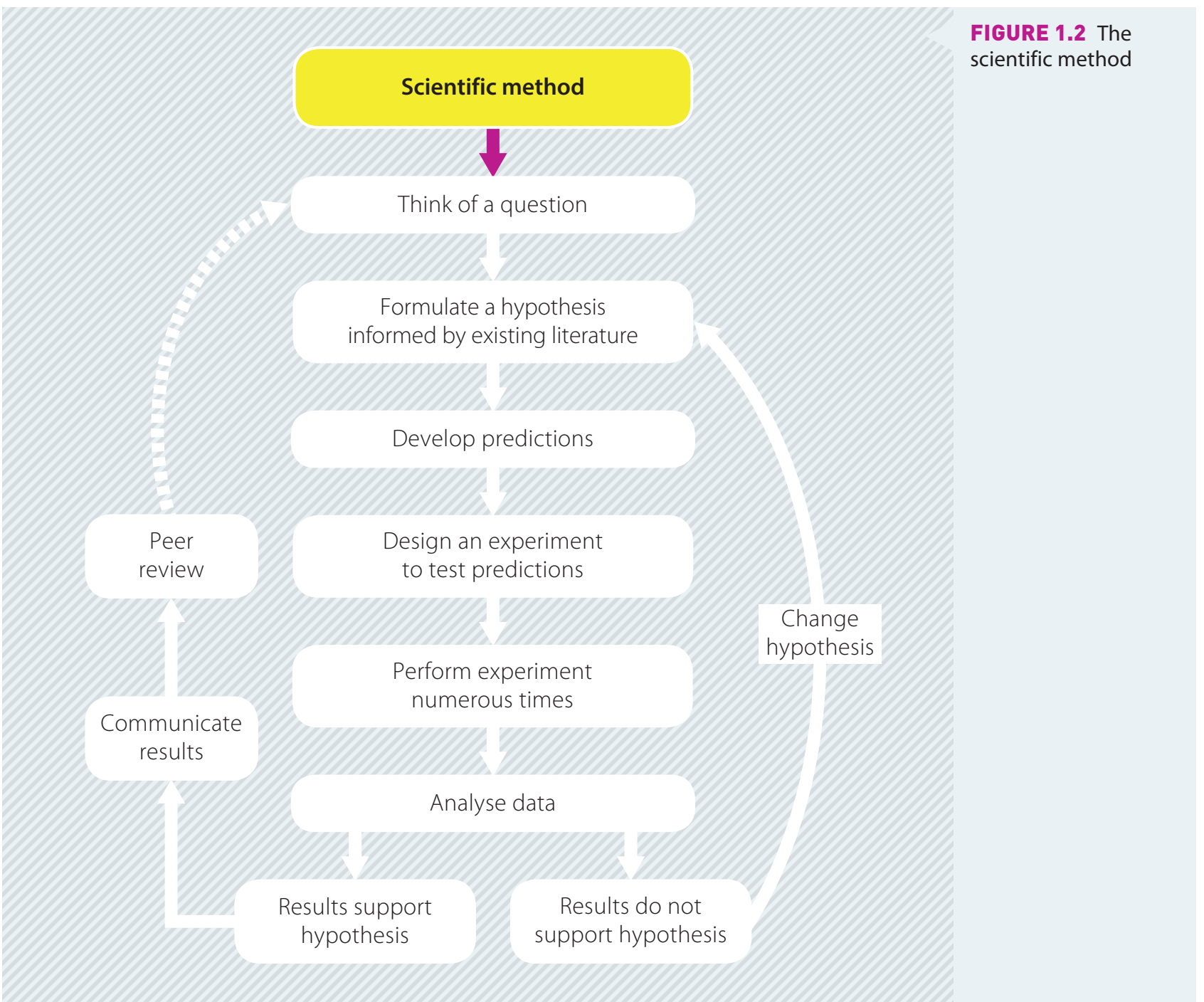
Photo AI
Last Updated Sep 24, 2025
Metal Reactivity Series Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Metal Reactivity Series quickly and effectively.
400+ students studying
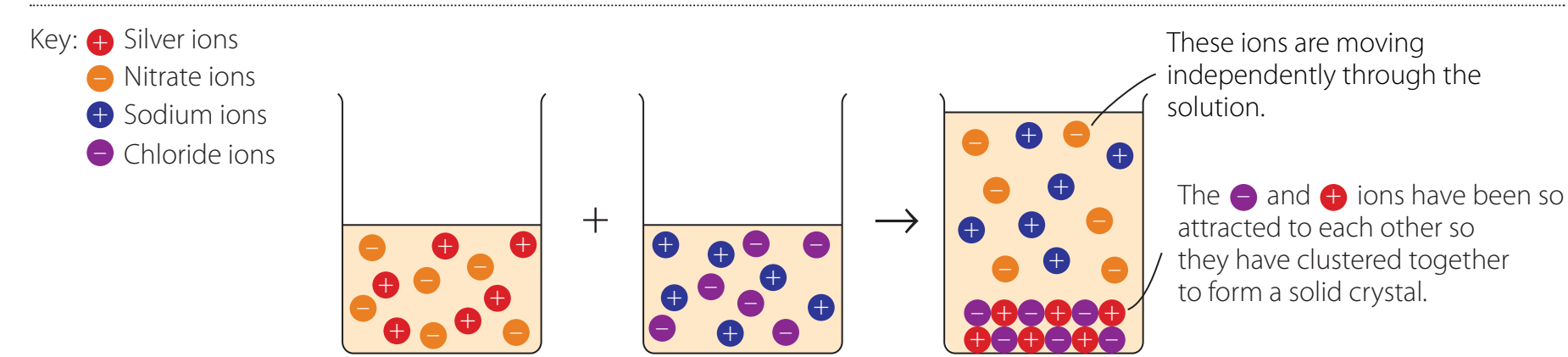
Metal Reactivity Series
Introduction to Metal Reactivity
Definition and Significance of Metal Reactivity
Metal Reactivity: The propensity of metals to release electrons and form positive ions. This behaviour is essential for facilitating chemical reactions.
Metal Reactivity: Metals release electrons to form cations, which is crucial in chemical processes.
Importance
- Integral for electrochemical applications, including advancements in battery technology.
- Influences the production of alloys and the longevity of metals.
Example: Understanding reactivity enables industries to choose corrosion-resistant materials, vital in fields like automotive manufacturing.
Factors Affecting Reactivity
Atomic Size and Electron Detachment
- Larger Atomic Size:
- Valence electrons are further from the nucleus.
- Easier electron extraction, enhancing reactivity.
- Highly Reactive Metals:
- Example: Potassium exhibits high reactivity due to its atomic size.
Electronegativity and Industrial Application
- Low Electronegativity:
- Facilitates easier electron release.
- Sodium in Electroplating:
- Benefits from low electronegativity, boosting its reactivity.
Trends in the Periodic Table
Periodic Trends
- Reactivity Increases Down a Group:
- Attributed to larger atomic size and enhanced shielding.
- Reactivity Decreases Across a Period:
- Due to heightened effective nuclear charge.
Expert Insights
- Down groups, larger atomic size and increased shielding lead to heightened reactivity.
- Across periods, increased nuclear charge draws electrons closer, damping reactivity.

- Diagram Explanation: Illustrates the effects of atomic size on reactivity trends.
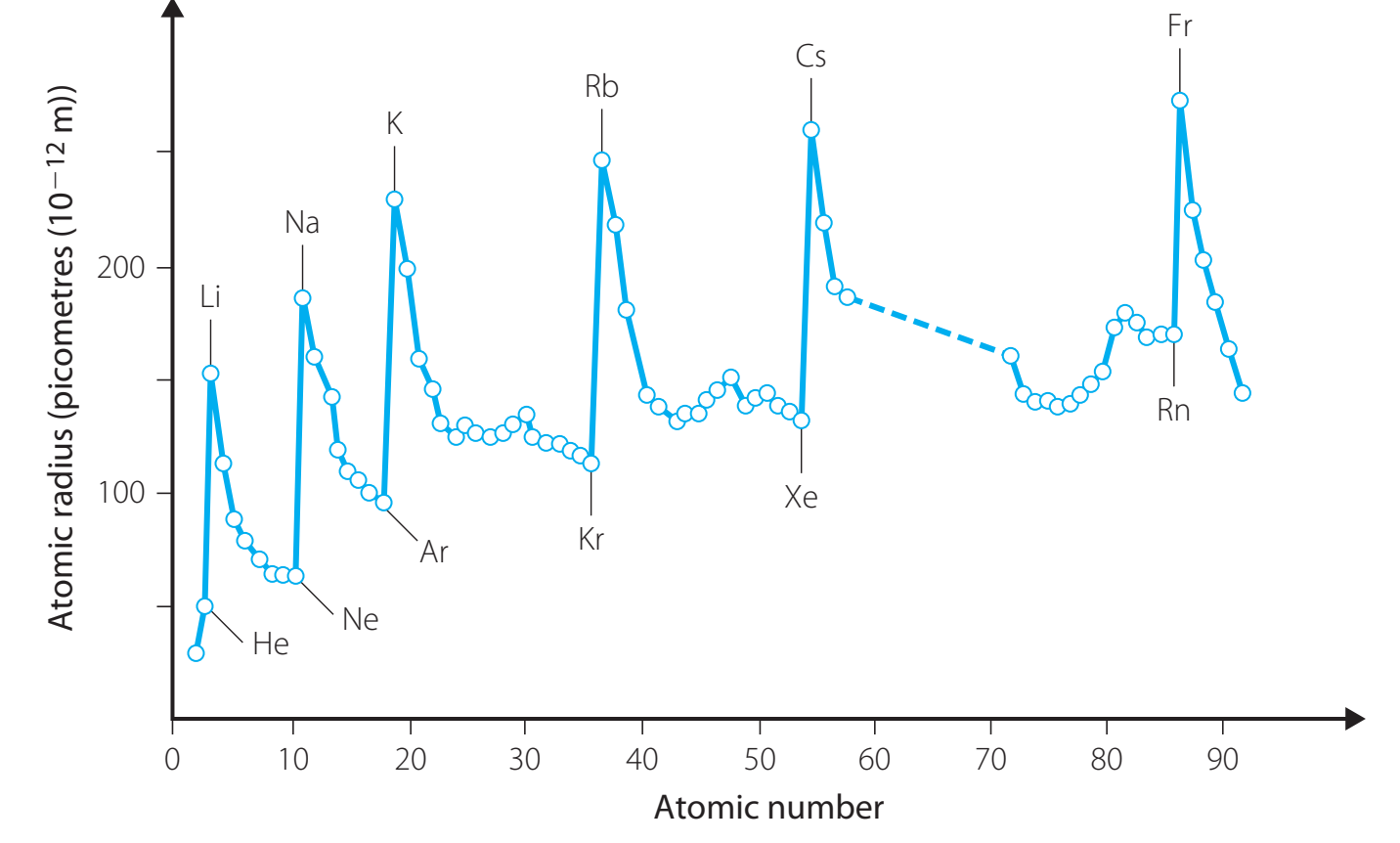
- Diagram Explanation: Depicts how electronegativity influences reactivity patterns in the periodic table.
Recognising these diagrams is essential for predicting characteristics of unfamiliar metals.
Common Misconceptions
Reactivity vs Corrosion
- Reactivity does not automatically mean rapid corrosion. Some metals react without quick corrosion.
- Crucial for sectors like construction to accurately assess corrosion resistance.
Practical Implications
- Misinterpretations can impact aerospace and automotive sectors.
- Proper understanding ensures optimal material performance in various conditions.
Introduction to the Reactivity Series of Metals
Reactivity Series: A classification of metals by their ability to displace others in reactions and their ease of electron loss.
- Importance:
- Aids in predicting metal reactivity.
- Facilitates comprehension of scientific patterns.
Purpose and Application
- Predictive Tool:
- Essential for anticipating the feasibility of chemical reactions, particularly displacement reactions.
- Example: Used in industrial processes, like employing carbon in a blast furnace for extracting iron, key in steel production.

- Reasoning for Metal Displacement:
- Metals higher in the series release electrons more freely.
- Can displace metals lower in the series from their compounds.
- Industrial Relevance: Crucial in metallurgy for obtaining pure elements, such as extracting iron during steel production.
Experimental Basis
Displacement Reactions
-
Follow these steps to understand displacement reactions:
- Immerse a zinc strip in a copper sulphate solution.
- Detect zinc displacing copper, forming zinc sulphate.
- Note the change in solution colour due to copper deposition.
-
Methods for Assessing Reactivity:
- Observe temperature changes during the reaction.
- Track gas evolution as an indicator.

-
Mnemonic Devices:
-
Mnemonic: "Please Stop Calling Me A Careless Zebra That is Learning How Copper Saves Gold"
- Mnemonic Breakdown:
- Please - Potassium
- Stop - Sodium
- Calling - Calcium
- Me - Magnesium
- A - Aluminium
- Careless - Carbon
- Zebra - Zinc
- That - Tin
- is - Iron
- Learning - Lead
- How - Hydrogen
- Copper - Copper
- Saves - Silver
- Gold - Gold
- Mnemonic Breakdown:
Mnemonics are crucial for examinations, enhancing recall and aiding memory retention.
Exceptions and Uncommon Behaviours
- Noble Metals like gold are less reactive but highly valued.
- Aluminium forms a protective oxide layer, masking its reactivity.
Expert Insights and Common Misconceptions
Expert Insights
- Acknowledge notable anomalies in expected metal behaviours.
Common Misconceptions
- Environmental Impact:
- Environmental factors, such as humidity, influence reactivity.
- Examples:
- Iron corrodes faster in humid settings.
- Metals corrode at varying rates in coastal versus inland environments.
Investigating Metal Reactions with Water
Safety Precautions
-
Essential PPE:
- Gloves: Provide protection from direct contact and chemical splashes.
- Goggles: Protect against potential eye exposure from splashes.
- Lab Coats: Shield from chemical burns due to spills.
-
Risk Management:
- Conduct thorough risk assessments prior to starting experiments.
- Adhere strictly to guidelines for managing and disposing of reactive metals.
Reminder: On their own, Alkali metals can react intensely with water; handle with care and avoid direct contact.
Procedure for Water Reactivity Tests
Understanding metal reactivity assists in analysing hydrogen gas production and other indicators.
-
Structured Step-by-Step Guide:
- Assemble precise materials: specified metal samples and glassware.
- Carefully introduce metal pieces to water.
- Methodically record hydrogen gas production and any noticeable changes.
-
Material Specifics:
- Use 100 ml of water and small (0.5g) metal pieces for controlled reactions.
Chemical Reactions and Observations
-
Sample Reaction:
- Explanation:
- (s): solid
- (l): liquid
- (aq): aqueous
- (g): gas
- Explanation:
-
Observation of Hydrogen Gas: Central to assessing metal reactivity.
Observing hydrogen production is crucial to understanding metal reactivity — note rate and extent of evolution with precision.
Common Misconceptions and Well-Recognised Trends
-
Misconceptions:
- Not every metal reacts violently with water. For instance, Zinc exhibits weak reactivity.
- Aluminium may appear non-reactive due to its protective oxide coating.
-
Recognised Trends:
- Reactivity ascends as you move down the group from Magnesium to Potassium.
Grasping these trends is key for exam preparation.

Visual Aids and Tables
- Utilise diagrams to conceptualise reaction setups and rates.
- Tables should distinctly highlight comparative data to support learning.

Student Engagement and Reflection
-
Prompt Questions:
- "How does hydrogen gas production correlate with your understanding of the reactivity series?"
-
Engage with hypothetical scenarios to test your understanding:
- "If a metal reacts slowly, what factors could be influencing this based on periodic trends?"
Integration with Academic Requirements
- Solve examples predicting metal reactivity based on periodic trends to connect classroom learning with practical applications like battery reactions.
- Embed thought exercises within the text to challenge and engage students effectively.
Introduction to Acid Reactions
Understanding metal-acid reactions is crucial across diverse industries. These reactions play essential roles in processes like hydrogen fuel production and managing safe chemical reactions of materials.
- Dilute Acid: Aqueous solution of an acid.
- Reactivity: The tendency of a metal to react with acids to form a salt and release hydrogen gas.
Hydrogen Evolution: Frequently observed as hydrogen gas is released during experiments.
This knowledge is applicable in real-world settings such as hydrogen fuel production and understanding corrosive processes in engineering.
Safety Protocols
- PPE Essentials: Always wear protective goggles, gloves, and lab coats in laboratory settings.
- Immediate Reactions: In the event of an acid spill, neutralise it with baking soda and rinse affected areas well.
- Scenario Hazards: Mishandling could lead to chemical burns and unsafe gas releases.
Always adhere to safety protocols to prevent serious lab accidents.

Experimental Procedure
Methodology:
- Precisely measure the metal.
- Add the acid cautiously, maintaining controlled conditions to avoid accidents.
- Observe and document alterations using a table for systematic recording.
Ensure conditions are established for safety before proceeding with experiments.
Chemical Equations and Observations
- Zinc Reaction:
- Iron and Magnesium: These metals can show increased reactivity levels.
- Be mindful of notable temperature changes and visible gas formation.
Place equations in boxed sections to ensure they stand out from the text.
Misconceptions and Clarifications
- Not all metals like copper react with acids due to their lower reactivity. Copper typically remains unchanged.
- Historical Perspective: Early scientific understandings were often constrained by misconceptions, but modern science has addressed many of these.
Visual aids can assist in distinguishing different metal reactivities.
Trends and Series Impact
Utilising the reactivity series allows prediction of likely reaction outcomes and rates:
- Practical Exercise: Form predictions using the series before executing experiments.
- Example: Examine how magnesium's position leads to rapid reactions versus less reactive metals.
The reactivity series provides predictive insights into chemical behaviours.
Mnemonic Call-Out Box
- Mnemonic: "Please Stop Calling Me A Cute Zebra I Think She Intends Loving Happy Cows Smartly Gently."
- Use this memory device to remember the metal reactivity series.

Implement the mnemonic to facilitate reactivity series recall and application during exams.
Reactions with Oxygen
Overview
Reactions with oxygen are indispensable in both chemistry and industry. Key outcomes include:
- Energy Release: Commonly observed during fuel combustion (e.g., candle flame).
- Corrosion: The gradual degradation of metals, like iron rusting.
- Protective Coating Formation: Some metals, such as aluminium, develop protective layers preventing further oxidation.
Key Concepts
- Oxygen Reaction: This occurs when a metal combines with oxygen, typically forming an oxide.
Oxygen Reaction: A reaction where a metal or substance engages with oxygen, forming an oxide.
- Examples:
- Rusting: Iron interacts with oxygen to form reddish-brown iron oxide.
- Combustion: A swift reaction producing heat and light.
Safety Measures
- Essential Safety Tips:
- Utilise Protective Gear: Critical to wear goggles and gloves.
- Assure Proper Ventilation: Necessary to avoid inhaling fumes.
- Stay Attentive: Must exercise caution with heated metals.
Procedure for Testing Metal Reactivity with Oxygen
-
Step-by-Step Guide:
- Prepare equipment: Collect various metals for testing under controlled conditions.
- Apply heat: Observe each metal's response to oxygen, paying attention to indicators like light emission or colour transitions.
-
Observational Table:
Metal Expected Observation Iron Formation of rust, displaying a reddish-brown colour. Copper Formation of black copper oxide, often in low visibility. Magnesium Burns with intense white light, forming white magnesium oxide. -
Visual Indicators:
- Highlight transformations like magnesium's bright light emission for clarity.
Chemical Reactions and Equations
- Balanced Chemical Equations:
- Magnesium and Oxygen:
Balanced Chemical Equation: An equation where the number of atoms for each element is consistent across both sides.
- Importance: Ensuring balance reflects mass conservation in reactions.
Specific Behaviours and Exceptions
- Protective Coatings:
- Aluminium: Forms a protective layer, unlike iron, which experiences full rusting upon continuous exposure.
Factors Influencing Metal Reactivity with Oxygen
-
Environmental Factors:
- Humidity: Promotes rusting in metals like iron.
- Temperature: Elevated temperatures boost reaction speeds.
-
Reactivity Trends:
- Larger atomic size and increased electronegativity are linked to greater reactivity.
Common Misconceptions
-
Not all oxides are reactive.
-
Copper is less reactive than iron and does not rust.
-
Clarification: Copper develops a stable oxide layer that inhibits further oxidation, unlike rust.
Definition and Redox Principles
Displacement Reactions: Reactions where a more reactive metal displaces a less reactive metal from its compound.
- Oxidation: The process of losing electrons by a chemical element.
- Reduction: The acquisition of electrons by a chemical element.
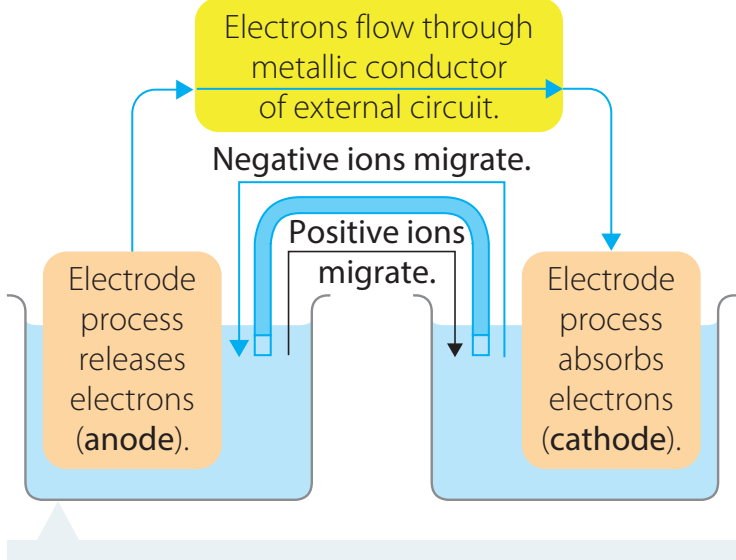
In displacement reactions, oxidation and reduction occur concurrently.
Role of the Metal Series
Metal Reactivity Series: A systematic guide for predicting displacement reactions by ordering metals based on reactivity.
- Mnemonic: Please Say Little Charlie Can Manage A Gigantic Zebra. I Tell You Later, However Correct Me Sincerely.

Experimentation and Step-by-Step Examples
Example: Iron in Copper Sulphate Solution
-
Step 1: Place iron nails into the copper sulphate solution.
-
Step 2: Notice: Copper deposits on nails, causing deposition.
-
Step 3: Conclusion: The solution lightens as iron sulphate forms.
-
Chemical Equation:
Notable Exceptions and Unusual Results
- Transition Metals: Form complexes exhibiting varied reactivity.
- Noble Metals: Typically resist displacement due to inherent low reactivity, despite their positions in the series.
Data Compilation and Conclusions
- Data Tables: Compare anticipated outcomes to real-world findings.
- Conclusion Guide: Offers steps to link experimental data back to the reactivity series.
Common Misconceptions
- Misconception: Mixing solutions automatically results in chemical reactions.
Always verify reactions by looking for evidence such as precipitate formation or colour changes.
Safety Considerations
Safety Protocols:
- Wear PPE: Including gloves and goggles to avoid accidents.
- Ensure Proper Ventilation: Reduces the risk of inhaling harmful gases.
- Handle Chemicals Carefully: E.g., copper sulphate, which can be hazardous if misused.

Overview of Data and Hypotheses
- Hypotheses: Direct practical experiments by forecasting outcomes based on existing data and observations.
- Knowledge of metal reactivity enhances safe and successful chemical experimentation.
Data Analysis: The activity of assessing data using analytical and statistical methods to uncover useful insights.
Using Experimental Data
Data Collection
- Importance in Understanding Metal Behaviour:
- Predict chemical reactions more reliably.
- Enhance experimental precision and efficiency.
- Foster safety in the laboratory.
Tables and Recording Observations
- Organise data with tables for clarity and order.
- Illustration of Data Progression:
| Step | Observation |
|---|---|
| Initial | Zinc placed in HCl |
| Reaction | Effervescence observed |
| Final | Zn dissolved, bubbles formed |
Tip: Employ digital spreadsheets for efficient data recording.
Creating Activity Series
Developing a Series
- Steps for Developing an Activity Series:
- Conduct tests to measure metal reactions.
- Note consistent observations.
- Arrange metals by measured reactivity.
- Example Scenario: Evaluating how iron behaves in acid determines its rank in a reactivity series.

Illustrative Examples
- Hypothetical Dataset:
| Metal | Reactivity Order |
|---|---|
| Magnesium | 1 |
| Aluminium | 2 |
| Iron | 3 |
| Lead | 4 |
Discrepancies, such as measurement errors, can impact results.
Hypothesis Formation
- Framework for Creating Hypotheses:
- Gather and scrutinise data on metal reactions.
- Detect reactivity patterns.
- Predict outcomes. For example, "Metal A will exhibit stronger reactions than Metal B based on data."
Common Hypothesis Formation Mistakes: Not grounding hypotheses in empirical data, neglecting outliers.
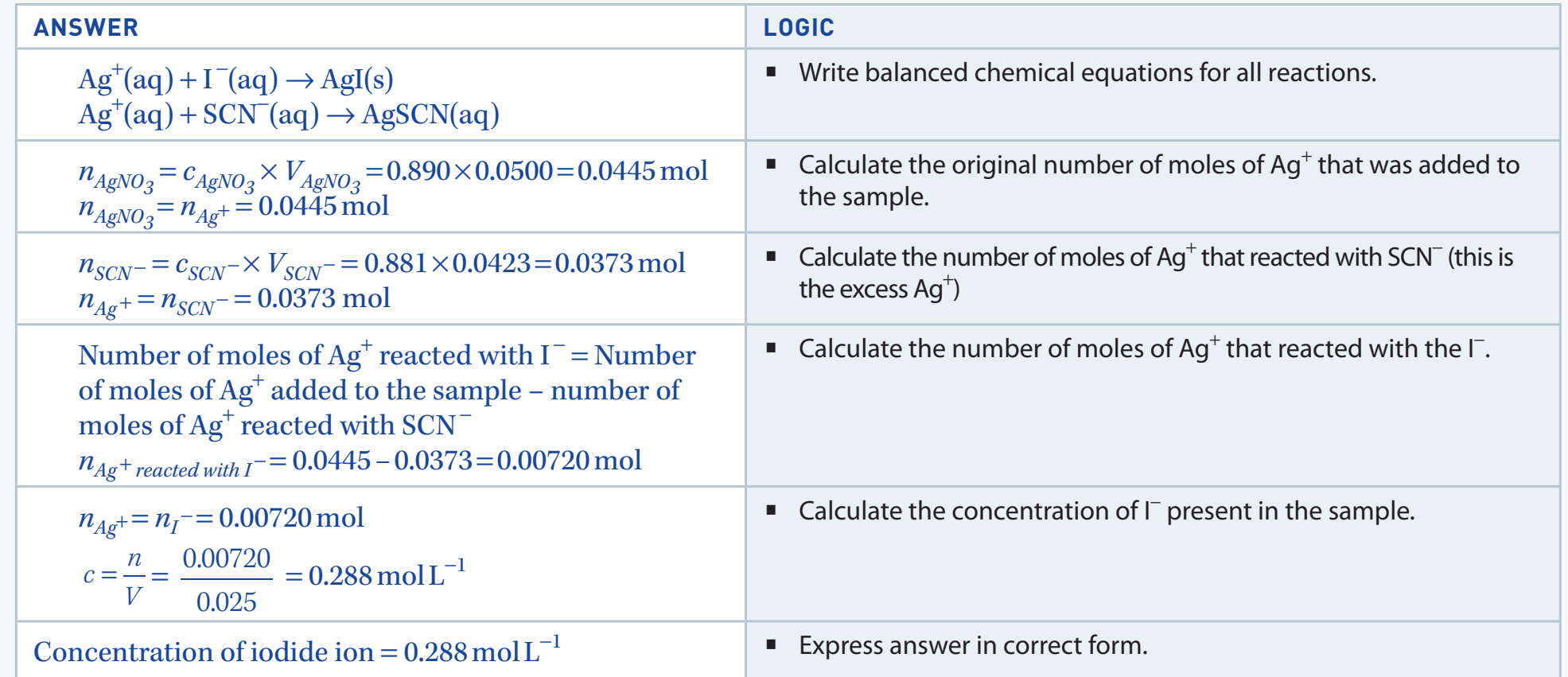
Quantitative Analysis
Understanding Stoichiometry
-
Stoichiometry: Involves calculating chemical equation quantities using reactants and products.
-
Example Calculation:
- Formula:
- Convert 0.5 moles of NaCl to grams:
Utilise calculators for stoichiometric calculations for accuracy.
Equations and Analysis
- Using simplified balanced equations helps in effectively predicting chemical reactions.
Statistical Tools
Data Analysis Methods
- Mean, Variance, Standard Deviation:
- Compute these statistics to understand data distribution and averages.
- Worked Example:
- Data Set: [10, 12, 23, 23]
- Calculate the mean, then use similar methods for variance and standard deviation.
Graph Creation
- Visualisation with R and Python:
- Use step-by-step guidance to generate graphs for enhanced data insight.

Accessible Summaries
- Summarising findings using visual aids ensures clarity.
- Effectively highlights trends or anomalies through charts and graphs.
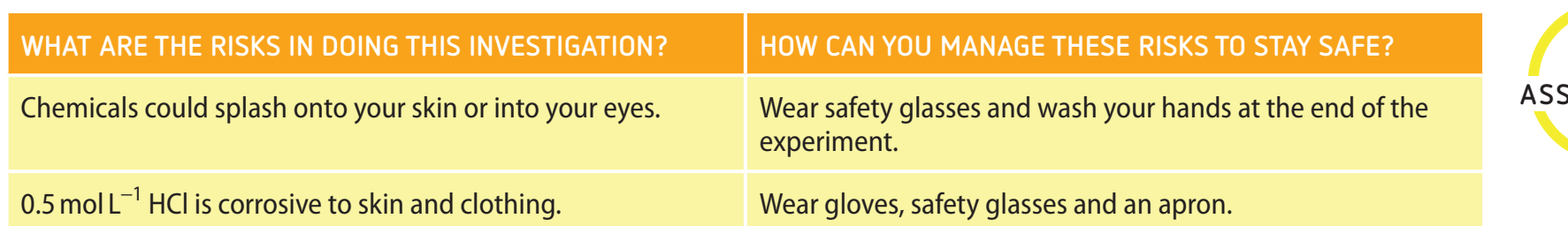
Safety Guidelines for Conducting Experiments
Conducting experiments with reactive metals mandates adherence to essential safety protocols to ensure laboratory safety. This guide provides structured instructions to promote safe practices.
Importance of Safety Protocols
- 1.1 Definition: Reactive Metals: Highly reactive metals like sodium and potassium that energetically react with substances like water and air.
- 1.2 Overview of Safety Concerns: Improper handling can cause explosions or fires.
- 1.3 Necessity for Safety Protocols: Proper procedures are vital to sustain safety and prevent harm in laboratory settings.
Personal Protective Equipment (PPE) Guidelines
- 2.1 Essential PPE:
- Lab coats and aprons: Protect attire and skin from spills.
- Safety goggles: Shield eyes during experimentation.
- Gloves: Choose between latex or heat-resistant gloves as necessary.
- Closed-toe shoes: Guard against spills.
- 2.2 Situational PPE:
- Utilise face shields to prevent exposure to splashes or vapours.
Quick PPE Tips: Always perform a fit check for goggles before starting an experiment. Determine any additional PPE requirements through assessment.
Experiment-Specific Safety Procedures
- 3.1 Water Reactivity:
- Use small quantities of metals to minimise the risk of vigorous reactions.
- Introduce substances gradually and utilise pipettes when feasible.
- 3.2 Acid Reactivity:
- Introduce acids slowly to prevent hazardous splashes.
- Utilise fume hoods to mitigate risks from volatile reactions.
- 3.3 Oxygen Reactivity and Heated Metals:
- Employ tongs for secure handling of heated metals.
- Ensure adequate ventilation to effectively manage gases and smoke.
Safe Experimentation Environment
- 4.1 Laboratory Setup:
- Ensure clear labelling of chemicals and proper storage to prevent misplacement.
- Maintain easy access to first-aid kits and emergency exits.
Always ensure lab preparedness before initiating any experiment. Confirm readiness of safety equipment like eyewash stations and first-aid kits.
Risk Assessment and Mitigation
- 5.1 Risk Identification:
- Undertake comprehensive risk assessments to pinpoint and mitigate potential hazards.
- 5.2 Mitigation Strategies:
- Anticipate risks and devise strategies for managing potential incidents.
- Establish clear emergency protocols, including appropriate use of first-aid and evacuation measures.
Use of Visual Aids and Callouts
- Infographic Reference:
- Utilise this infographic as a visual checklist for ensuring appropriate PPE usage.
- Laboratory Setup Diagram Reference:

- Refer to this diagram for optimal laboratory arrangement before any experimental activities—align with your preparatory steps.
Glossary
- Reactive Metals: Highly active metals like sodium, marked by vigorous reactions with water and oxygen.
- Fume Hood: A ventilated chamber used in laboratories for safely conducting experiments involving hazardous materials.
By adhering strictly to these guidelines, students can confidently and safely conduct experiments, establishing a solid foundation in safety awareness for successful laboratory experiences.
500K+ Students Use These Powerful Tools to Master Metal Reactivity Series For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
279 flashcards
Flashcards on Metal Reactivity Series
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Metal Reactivity Series
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes18 questions
Exam questions on Metal Reactivity Series
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Metal Reactivity Series
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Metal Reactivity Series
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Metal Reactivity Series you should explore
Discover More Revision Notes Related to Metal Reactivity Series to Deepen Your Understanding and Improve Your Mastery
